Introduction
Tilapia farming is the second most significant freshwater fish industry globally, following carp (FAO 2020). Currently, worldwide Nile tilapia production grew from 700,000 in 2010 to 850,000 tons in 2020 (FAO 2020). Principal Nile tilapia producers are China, Indonesia, Egypt, and the Philippines, with Brazil and Mexico emerging as important producers in the Americas (FAO 2020). In 2017, Mexico reported a total of 4,634 Nile tilapia farms operating throughout the country, collectively generating an annual production of 117,806 t. The states with the highest production are Chiapas, contributing 30,912.11 t, following by Jalisco (8,178.08 t), and Nayarit with (7,618 t) (INAPESCA 2018).
Nevertheless, the elevated stocking densities required to achieve current Nile tilapia production levels in Mexico have given rise to various parasitic agents (bacteria, viruses, and other parasites). Streptococcosis has emerged as a prevalent bacterial disease in Nile tilapia production world systems, as documented by Pretto-Giordano et al. (2010), Delannoy, Samai, and Labrie (2021), Zhang et al. (2021), Delphino, Leal, Gardner, et al. (2019), and Delphino, Joshi, and Alvarez (2022). Since its initial record in Nile tilapia in 2009, streptococcosis has become the predominant pathogen, accounting for over 90% of detected and isolated pathogens in aquaculture. This has resulted in mortality rates ranging from 30 to 90% on a global scale (Sheehan, Lee, Wong, et al. 2009; Liu, Ye, Liu, et al. 2018). In Nile tilapia farming, streptococcosis outbreaks, are mainly associated with Streptococcus agalactiae group B and S. iniae. Economic losses can reach 23 billion US dollars in countries like the United States, Israel, Japan, Kuwait, Thailand, Mexico, Honduras, and Brazil (Evans, Klesius, Gilbert, et al. 2002; Evans et al. 2006; Suanyuk et al. 2008; Mian et al. 2009; Delannoy, Zadoks, Lainson, et al. 2012; Delannoy, Samai, and Labrie 2021; Li et al. 2016; Zheng, Wu, Hu, et al. 2018).
In Malaysia and Mexico, mortality rates exceeding 50% have been recorded during acute infections, typically occurring within 3 to 7 days post infection. Alternatively, the rates can be lower but still result in a sustained daily mortalities during chronic infections (Zamri-Saad et al. 2014; Ortega, García, Irgang, et al. 2018). In Malaysia, Nile tilapia weighing between 300 to 400 grams experienced mortalities of up to 60% due to S. agalactiae (Zamri-Saad et al. 2014). Floating cages in Brazil reported mortality rates ranging from 25 % to 35 % (Leal et al. 2019), while in China, rates ranged from 20 to 30% (Ye, Li, Lu, et al. 2011). In Northern Africa, cultivation ponds reported mortality rates ranging from 6 to 14% (Delannoy, Samai, and Labrie 2021). Regarding S. iniae, mortality rates from 30 to 50% have been observed in Iowa (Rahmatullah, Ariff, Kahieshesfandiari, et al. 2017), and in Mexico, mortality rates of 68 and 80% were documented in Oreochromis aureus production (Ortega, García, Irgang, et al. 2018).
The overall scenario appears discouraging, as Streptococcus spp. has already become well-established and widely distributed across production systems in nearly 10 states of the Mexican Republic (Table 1). Despite the significance of Nile tilapia culture in Mexico, the scarcity of reports regarding the status of this disease is notable.
Given the substantial potential for losses associated with streptococcosis, it is imperative to enhance our understanding of this disease in Mexico. This will support the proposal of sanitary control strategies for aquaculture facilities and the development of biosecurity programs, thereby reducing disease incidence and mitigating economic losses and health risks. In Mexico, comprehensive information on various pathological aspects of this disease is lacking, including data about its prevalence, geographical distribution, and the associated economic losses resulting from its growing presence. Therefore, this study aims to describe and analyze the histopathological damage, economic losses, and clinical manifestations of streptococcosis in a commercial farm in Campeche, Mexico.
Materials and Methods
Field and laboratory work
A follow-up study designed to elucidate Streptococcosis infection was carried out in an intensive farm located in Chulbac, Chiná, in the municipality of Campeche during May 2023. A total of 30 Nile tilapia (Oreochromis niloticus) with sign such as eroded fins, injured eyes, exophthalmia, ulcers, ascites, external lesions and desquamation were collected using a 2 m diameter cast net with a 2.5 cm mesh from a production tank. The fish collected were kept in tanks with artificial aeration and transported to laboratory. Fish were euthanized with benzocaine (100 mg/l) until opercular movements ceased (Fabiani et al. 2013). Each specimen was measured to obtain the total length (TL, cm), and total weight (W, g). The fish had an average weight of 280 g, ranging from 210 to 340 g, and an average total length of 17.50 cm, ranging from 15.34 to 19.53 cm. The genomic DNA of Streptococcus agalactiae was validated for one-step or real-time PCR (qPCR) in concurrent research conducted by Castillo-Avila et al. (2024); however, the serotype was not determined.
Histopathology procedures
Nile tilapia organs (brain, liver, kidney, muscle, spleen, stomach and intestine) were removed and fixed in 10 % neutral-buffered formalin during 72 h prior to processing using standard protocols for histology. Organ sections were dehydrated in 70, 80 and 96% ethanol baths, cleared with xylene, and embedded in paraffin wax, and sectioned at 5 µm on a Leica™ microtome (Mumford et al. 2007; Igeh and Avenant-Oldewage 2020). Slides were left overnight in an oven at 40°C to ensure adhesion. Subsequently, sections were stained with haematoxylin and eosin (H&E); some sections were stained with Ziehl-Neelsen and Fite–Faraco stains for acid-fast bacteria and examined under a light microscope (Igeh and Avenant-Oldewage 2020). Digital images were obtained using a Leica Digital Camera MC 120 HD and capture software.
Health condition by evaluation of histopathological index
To evaluate the health condition of Nile tilapia, the organ tissues alterations of each fish were analysed using the histopathological index proposed by Schwaiger et al. (2004). Additionally, the prevalence of each lesion per fish was calculated and expressed as percentage. According to the semi-quantitative method described by Schwaiger et al. (2004), histological alterations in tissue organs received damage severity scores. Following scores were used: 1) mild alterations or focal process in gills, 2) moderate alterations or multifocal, and 3) severe alterations or diffuse process. Moreover, the mean value of histological alteration was established for each fish into three categories mild (0.1–1.0), moderate (1.1–2.0), and severe (2.1–3.0) (Steckert et al. 2018). The histological alteration was analysed using the nonparametric Kruskal–Wallis statistical test (p<0.05).
Economic losses analysis
A database containing information about monthly biomass losses caused by S. agalactiae from January to December 2022 provided by the fish farm owner was analyzed graphically. Prevalence, defined as the percentage of infected hosts among those tested, was assessed to measure the impact on production (Bush et al. 1997). The gross economic impact was estimated based on 10,000 interactions using a Monte Carlo simulation based on the following simple parameters (Bezerra et al. 2022): total losses of tilapia by S. agalactiae in one year (30,740 kg), minimum and maximum selling price by tilapia kg (80 – 120 Mexican pesos), and minimum and maximum production cost of 1 tilapia kg (45 – 55 Mexican pesos) (Personal communication, Dr. Fernando V. Iriarte-Rodriguez, Juárez Autonomous University of Tabasco – Aquaculture Program).
Results
Clinical manifestations of collected Nile tilapia
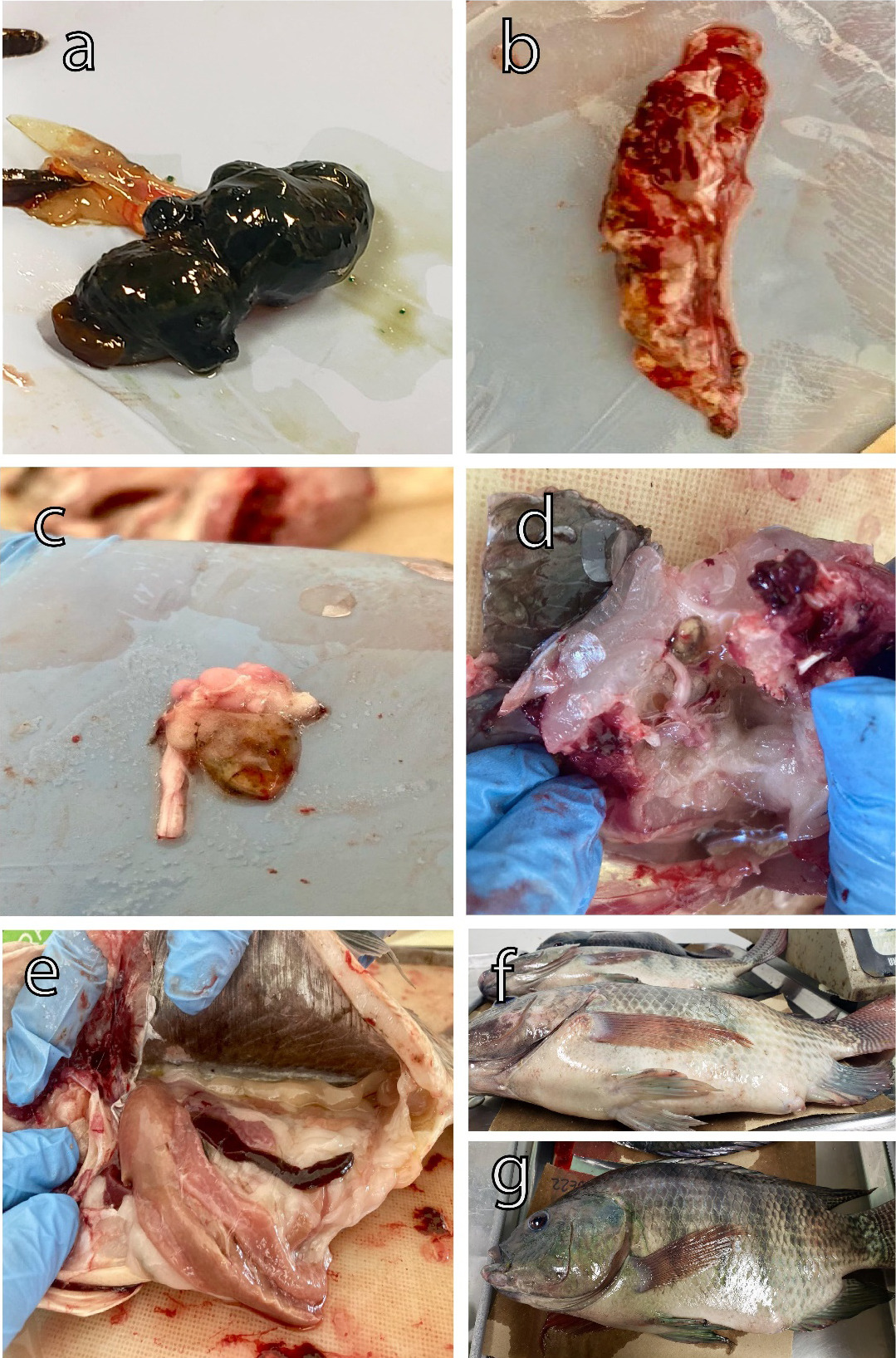
Outbreaks of S. agalactiae were detected in grow-out ponds in a Nile tilapia farm, reaching an 80% of prevalence, which resulted in high mortality and morbidity rates. Necropsies on the fish revealed classic signs of streptococcosis, including bilateral exophthalmia, fish exhibited protruding or swollen eyes (Figure 1a). Spleen with nodules typically showed focal areas of inflammation and bacterial proliferation within the spleen tissue (Figure 1b). The hemorrhagic brain was characterized by internal bleeding within the brain tissue (Figure 1c). Nervous cord with hyperplasia is characterized by the growth of nerve tissue, particularly in the spinal cord region, which can lead to neurological dysfunction (Figure 1d). Hepatomegaly and splenomegaly were observed post-mortem examination of affected fish (Figure 1e). Ascites accumulation of fluid was observed in the abdominal cavity, resulting in swelling or bloating of the abdomen in affected fish (Figure 1 f,g).
It is important to note that certain batches of fish did not exhibit any obvious disease signs before experiencing significant mortality. However, a high occurrence of secondary lesions (myositis and caudal fin deformities, spinal cord curvature, necrosis in and on the muscle) bleeding at the base of the fins, and tail fraying was observed during the necropsies of affected organisms, indicating a chronic pattern (Figure 2a-e). Additionally, some fish exhibited significant hemorrhaging on the lips and mouth, severe congestion, and congested kidneys. Upon dissection of the fish, we observed the presence of bloody, hemorrhagic ascitic fluid in the abdominal cavity, which appeared dark in color (Figure 2d).
Histopathological examination
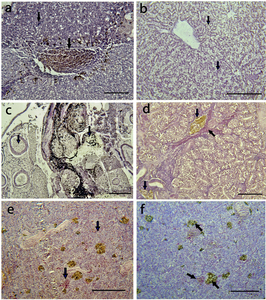
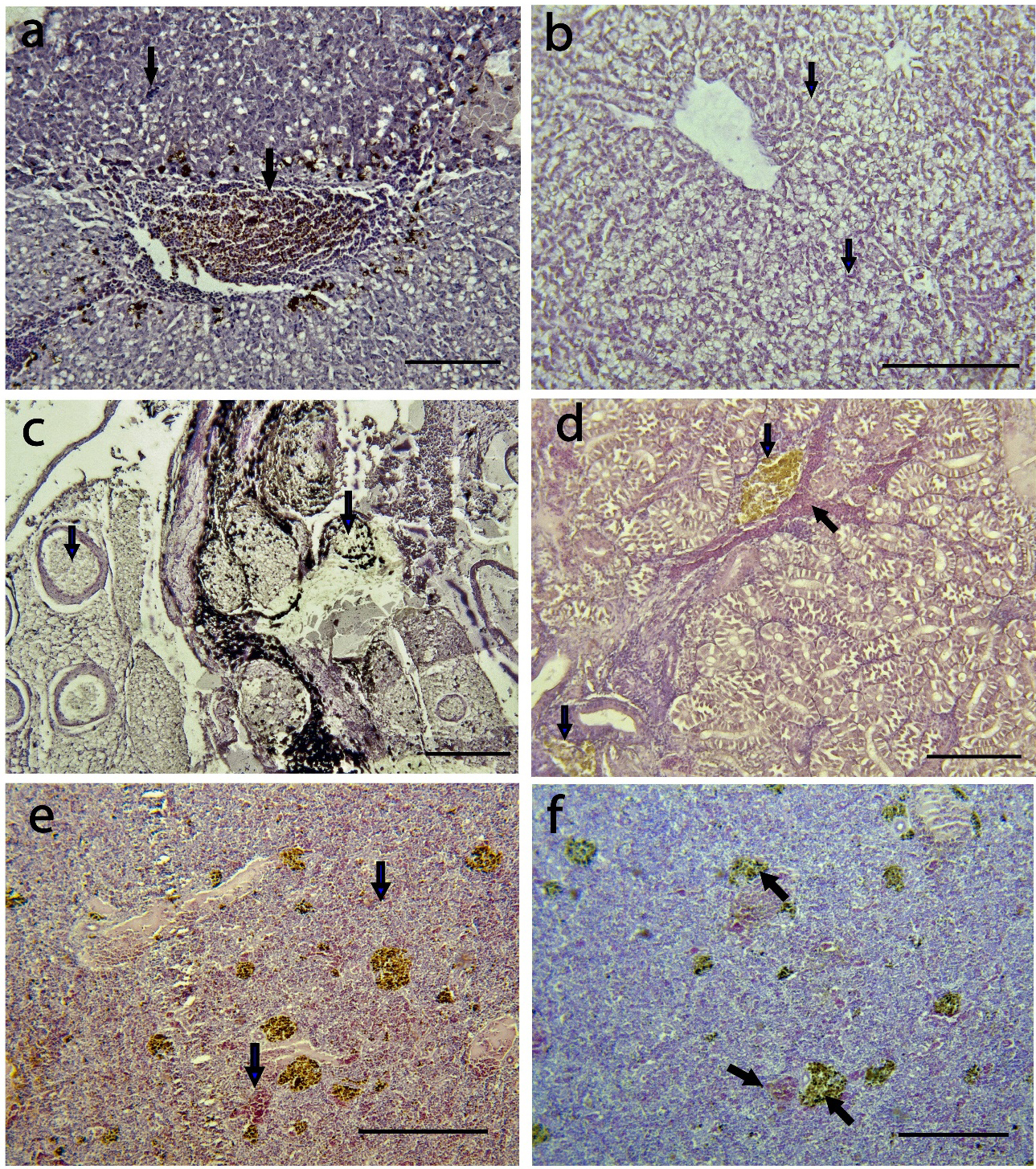
The brain, liver, kidney, muscle, spleen, stomach, and intestine of Nile tilapia infected with S. agalactiae displayed several degrees of histological alterations (Table 2). The histopathological examination of the liver tissue revealed the presence of melanomacrophage pigment (indicated by an arrow in Figure 3a), hemorrhage, and substantial eosinophilic infiltration surrounding the blood vessels. Additionally, there was evidence of edematous perivasculitis that progressed to congestion, thrombosis, and necrosis in the portal blood vessels (Figure 3a). Furthermore, vacuolation in hepatocytes was observed, along with lipid accumulation (indicated by an arrow in Figure 3b).
In the kidney, lesions were observed in both the excretory and hematopoietic tissues, accompanied by melanomacrophage activation (indicated by an arrow in Figure 3c). Additionally, there was evidence of lymphocyte depletion, as well as hemorrhage and necrosis (indicated by an arrow in Figure 3c, d).
In the spleen of the Nile tilapia, vascular congestion surrounded by inflammatory cell infiltration was observed (Figure 3e). Additionally, thrombosis of the splenic blood vessels was evident, along with an increase in the melanomacrophage center in the splenic parenchyma (Figure 3f).
The muscle displays pigmentation attributed to the presence of melanomacrophages and the formation of granulomas (indicated by an arrow in Figure 4a, b). The brain exhibits signs of encephalitis along with vascular congestion (indicated by an arrow in Figure 4c, d). In the intestine mucosa, showed moderate eosinophilic infiltrates, necrosis (indicated by an arrow in Figure 4e), and inflammatory cell aggregation (indicated by an arrow in Figure 4f).
Histopathological examination of the organ tissues revealed morphological changes in the tissue of all fish. The organs with most severe damage were liver, kidney and spleen (Table 2). The most prevalent histopathological damage reported in the fish was melanomacrophage pigment, granulomas, vacuolar degeneration, hemorrhage (Table 2). Melanomacrophage pigment and granulomas had severe damage with an alteration mean values of 2.81±1.90 and 2.3±1.85 respectively. While lymphocyte infiltration showed mild damage with an AMV of 0.24±0.19 (Table 2).
Economic losses analysis by Streptococcus agalactiae
In the first three months, tilapia harvests display an increasing trend, reaching maximum values in February at 80 tons and in March at 79 tons. Following these months, production declines by approximately 50%, reaching its lowest levels in November and December, with 16 tons each month (Figure 5a). The quantity of fish loss due to muscular necrosis exhibits a similar trend at the beginning of the year, with a maximum loss of 5,076 kg recorded in January and 4,302 kg in October (Figure 5a). Prevalence remains stable from January to June, reaching its highest rates in August and October, at 88% and 89%, respectively (Figure 5b).
Based on the Monte Carlo simulation the total annual gross economic loss due to muscle necrosis often referred to by producers as “fish with lumps,” averaged $1,537,671 ± 34,2587 Mexican pesos (76464.53 ± 17036 USD). The Monte Carlo model estimated the minimum and maximum losses ranging from 299,302 to 2,824,909 Mexican pesos (14,883 – 140,475 USD) (Figure 6).
Discussion
This study represents the first report detailing the economic losses and describing the lesions and clinical changes caused by S. agalactiae infection in an intensive farm in Campeche. The aquaculture health committee of the state of Campeche (CESAICAM) reported the presence of S. agalactiae for the first time in 2001, specifically in the northeast of Ciudad del Carmen. Additionally, the current paper represents the first record of S. agalactiae in recirculation systems in the central region of Campeche state.
In Mexico, there is currently no available data to ascertain when or how Streptococcus spp. was introduced. The situation appears concerning, as these bacteria have become well-established and are widely distributed across nearly ten states in the Mexican Republic (Table 1). The economic impact of streptococcosis at the national level could be significant, as evidenced by the mortality rates, product loss, and high treatment costs observed on the studied farm.
The most prevalent clinical signs during the acute phase of the infection in production systems include swimming abnormalities, anorexia, corneal opacity, unilateral or bilateral exophthalmia, skin ulcers, abscesses, and hemorrhages on the skin, jaw, and at the base of the caudal peduncle (Anshary et al. 2014; Iregui et al. 2014; Sun, Fang, Ke, et al. 2016; Yi, Wang, Li, et al. 2019; Zhang 2021). Additionally, abdominal distension, spinal cord curvature, hemorrhagic ascites, and poor body condition are observed, all of which are attributed to bacteria of the Streptococcus genus (Zhang 2021). Hemorrhagic and inflammatory reactions are commonly observed in affected organs such as the eye, liver, spleen, kidney, and brain (Rahmatullah, Ariff, Kahieshesfandiari, et al. 2017; Ortega, García, Irgang, et al. 2018; Alazab, Sadat, and Younis 2022). Similarly, Hernández, Figueroa, and Iregui (2009) demonstrated that S. agalactiae exhibits a preference for infecting specific organs, as the brain, eyes, and heart (with 71, 44, and 37 %, of frequency respectively). M. Hernández-Hernández et al. (2023) observed lesions that included exophthalmia, ascites, and granulomas at the base of the tail with pus accumulations.
However, subclinical, and chronic infections in adult Nile tilapia have been consistently detected. For instance, yellow or dark red nodules in the musculature near the vertebrae, myositis, and deformities of the caudal fin are evident (Sun, Fang, Ke, et al. 2016; Zhang et al. 2021). These subclinical findings likely indicate an adaptation by the bacterium to environmental factors characteristic of production systems, such as osmotic pressure, acidity, elevated levels of nitrogenous waste, and indiscriminate antibiotic use (Evans et al. 2009).
The histopathological findings were similar as reported in recent studies that indicated that infected fish with streptococcal disease exhibited multiple pathological conditions of tissues internal organ mainly the presence de granulomatous reaction, melanomacrophage pigment, vacuolar degeneration, granular eosinophilic cells with evident bacterial bodies (Alsaid, Hassan, Mohd, et al. 2014; He, Huang, Wang, et al. 2017; Laith, Ambak, Hassan, et al. 2017). Melanomacrophage were the most apparent damage histologic affected in organs in livers and spleens of fish infected with bacteria. The melanomacrophages have the function of eliminating, detoxifying, or recycling waste materials from erythrocytic and cellular metabolic processes. Additionally, melanomacrophages play a crucial role in the response to foreign entities, such as infectious agents or immune conditions (Franco-Belussi and de Oliveira 2016).
Several risk factors have been associated with the occurrence of streptococcosis outbreaks (Zhang 2021). High temperatures can enhance the pathogenicity of S. agalactiae in Nile tilapia. Zhao, Zou, Han, et al. (2023) reported higher mortality rates (80 %) at temperatures of 31°C compared to those exposed to 22°C (10 %) in fish infected with S. agalactiae. M. J. Hernández-Hernández et al. (2012) reported an 80% prevalence of Streptococcus spp. in 10 production units at Malpaso Dam, Chiapas. Additionally, a mortality rate of 50%, along with clinical signs in 60% of the cases. Also, an association was identified between low oxygen levels ranging from 5 to 6.5 mg/l and temperatures exceeding 31°C with the incidence of Streptococcus spp. Poor sanitary management was observed on the farm where the fish were collected. This included elevated temperatures, high stocking densities, wastewater channels situated close to the farm, dead fish, insufficient disinfection of fishing equipment and ponds, and inadequate water changes (Figure 7a-d).
In Mexican aquaculture, suboptimal management practices within production systems are common and may contribute to the increased presence and spread of pathogens. It has been consistently observed that the movement of fish for commercial purposes without the implementation of proper sanitary measures stands as the primary risk factor for the dissemination and introduction of both S. iniae and S. agalactiae (Amal et al. 2013; Paredes-Trujillo and Mendoza-carranza 2022). Notably, in Mexico, it is common practice to sell fingerlings without the issuance of sanitary certificates, coupled with the inadequate sanitary measures employed by many production farms. This combination promotes recurrent reinfections of the disease. It is imperative to identify and understand the routes and dynamics through which Streptococcus spp. enters in Nile tilapia farms.
Horizontal transmission appears to be the prevailing mechanism for the dissemination of Streptococcus spp. (Kim et al. 2007; Amal et al. 2013). It is well-established that certain elements, such as contaminated water, infected fingerlings, direct contact with deceased fish, high fish stocking densities, and fluctuations in environmental variables (elevated salinity, low dissolved oxygen levels, and high nitrogenous waste concentrations), can heighten the susceptibility of Nile tilapia to infection (Amal et al. 2013; Zamri-Saad et al. 2014). Additionally, infections can result from the excretions of diseased fish and cannibalism (Kim et al. 2007). Furthermore, there is substantial evidence supporting the possibility of vertical transmission as a route for this bacterial disease to infect the offspring (Pradeep, Suebsing, Sirthammajak, et al. 2016). Nevertheless, it remains essential to continually assess the specific risk factors to each production unit to confirm these introduction pathways, with a particular focus on the context of Mexico.
Mexico is situated in the subtropical and tropical zones, where temperature plays a crucial role in infection dynamics. It is widely recognized that elevated temperatures between 30 and 35 °C are closely linked to the occurrence of S. agalactiae outbreaks in Nile tilapia (Evans et al. 2009; Mian et al. 2009; Amal et al. 2013; Paredes-Trujillo and Mendoza-carranza 2022). Poor management practices, such as low water replacement and uncontrolled food supplies, can lead to increased nitrogenous waste, particularly ammonia. Elevated ammonia levels (0.24 mg/l, >10 mg/l, 0.3 mg/l) have been linked to Streptococcus agalactiae, which may, in turn, be associated with weakened host defenses due to stress (Evans et al. 2006). Water quality plays a pivotal role in maintaining the well-being of cultured fish populations, enhancing biomass production, and supporting their natural resistance to diseases (Amal et al. 2013). Variations in physicochemical parameters and contamination resulting from suboptimal management practices and inadequate water control can create conditions that favor pathogen survival, consequently affecting the immune response of fish (Amal et al. 2013).
The sanitary management of Nile tilapia is fundamental to the production process and must be conducted according to standardized procedures throughout all phases, beginning with the management of fingerlings and breeders (including genetic improvement and reproduction), as well as ensuring water quality and sanitary practices. Designing an effective biosecurity program requires a thorough risk analysis of fish diseases. This process includes identifying potential sources of pathogens, understanding transmission routes, and evaluating the susceptibility of the cultured species. The concept of biosecurity measures in Nile tilapia culture in Mexico has not yet been fully implemented. An ongoing concern in Mexican aquaculture systems is the excessive use of antibiotics, which are often administered by producers as the first line of treatment for Streptococcus spp. infections, without prior and accurate laboratory diagnosis. Precise identification of the bacterium is essential for determining the correct dosage and treatment. Improperly administered antibiotics not only pose significant risks of antibiotic residues but also contribute to the development of antibiotic resistance in the bacteria (Amal et al. 2013).
Conclusions
Streptococcosis in Nile tilapia represents a significant challenge in Mexico, marked by a lack of comprehensive historical data and an incomplete understanding of its current dynamics. There is limited documentation regarding the presence of Streptococcus spp. in major Nile tilapia production regions.
The economic losses linked to streptococcosis on Nile tilapia farms in Campeche raise serious concerns for farmers. Histopathological damage, chronic infections, and targeted mortalities are notable characteristics of the disease, further alarming the overall health of the fish population.
Given this context, it is imperative to intensify scientific research to gain precise insights into the strains present and their transmission dynamics, as well as to propose effective prevention and eradication measures. Additionally, urgent educational initiatives must be undertaken to inform producers about disease prevention, control, and identification. It is also essential to establish early intervention protocols involving producers and collaboration with national and state aquaculture health organizations.
Acknowledgments
This research was supported by the Consejo Nacional de Ciencia y Tecnología (CONAHCYT) under posdoctoral grant [253392]. Thanks are extended to the Laboratorio de Sanidad Acuícola, Instituto de Ecología, Pesquerías y Oceanografía (EPOMEX) de la Universidad Autónoma de Campeche. Thanks to Rodolfo Rodríguez del Río for facilitating the acquisition of the biomass data, Ana Delia Cu Escamilla, Ricardo Ávila Castillo. Thanks to owner of the Nile tilapia farm in Campeche for providing biomass loss data, as well as for sample collection.
Funding statement
This research was funded by Universidad Autónoma de Campeche (www.uacam.mx), with internal funds. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors have no conflict of interest to declare regarding this publication.
Ethical statement
We confirm that the animals in this study do not suffer any animal abuse. Approved by the research ethics committee of ECOSUR (Comité de Ética para la Investigación de El Colegio de la Frontera Sur) official letter number CEI-05-10-2023 that followed the International Guiding Principles for Biomedical Research Involving Animals.
Author contributions
Conceptualization: A Paredes-Trujillo.
Data curation: A, Paredes-Trujillo, M, Mendoza-Carranza.
Formal analysis: A, Paredes-Trujillo, M, Mendoza-Carranza.
Funding acquisition: A, Paredes-Trujillo.
Investigation: A, Paredes-Trujillo, M, Mendoza-Carranza.
Methodology: A, Paredes-Trujillo, M, Mendoza-Carranza.
Resources: A, Paredes-Trujillo, M, Mendoza-Carranza.
Supervision: A, Paredes-Trujillo, M, Mendoza-Carranza.
Writing – original draft: A, Paredes-Trujillo.
Writing – review and editing: M, Mendoza-Carranza.




_production_(left_axis)_and_losses_by_*streptococcus_agalactiae*_(right_axis)_in_a_n.jpg)




_production_(left_axis)_and_losses_by_*streptococcus_agalactiae*_(right_axis)_in_a_n.jpg)

