Introduction
In 2023, global fish production reached approximately 185 million tonnes, with 94 million tonnes derived from aquaculture production, accounting for 51% of the total, a significant increase from 25.7% in 2000 (FAO 2023). Human fish consumption increased at an average annual rate of 3.1% from 1961 to 2017 reaching 150 million tonnes with production reliance on fishmeal and fish oil remaining stable at about 19%. So, aquaculture is a rapidly growing food-producing sector, aquatic products are highly traded, with trade boosted by increased consumption, improved storage, preservation, transportation and liberalization policies. Fish production, however, faces numerous challenges, including climate change, poor water quality, high stocking densities, and infectious diseases (Martos-Sitcha et al. 2020). These issues are further compounded by stringent environmental regulations and decreasing availability of optimal production sites (FAO 2023). By 2050, the global population is projected to reach 9.7 billion (UNDESA 2024), but with 10% undernourished or suffering from chronic hunger (FAO 2023). Simultaneously, rising socio-economic and environmental concerns have fuelled growing interest in fish welfare, aiming to enhance production and minimize disease-related mortality. Parasites have a major impact in aquaculture, affecting farm production, sustainability, and economic viability due to the costs associated with control and management of infection and stock loss (Shinn et al. 2005); they are the ‘weeds of aquaculture’ (Cable and Harris 2002).
Traditional preventative and effective chemicals extensively used in the past, such as malachite green and formalin, were banned due to their detrimental impact on humans, animals and the environment (Srivastava, Sinha, and Roy 2004). Anti-helminthics, like praziquantel, have other adverse effects including altering food fish palatability (Hirazawa et al. 2004; R. Williams et al. 2007). Even though anti-parasitic drugs administered orally with food, such as praziquantel and trichlorfon, can also be applied as bath applications (Dunn et al. 1990), they too are linked with environmental and host toxicity, development of host resistance and low palatability (Hirazawa et al. 2004; R. Williams et al. 2007). Vaccination can significantly reduce disease outbreaks in aquaculture, typically administered via intraperitoneal injection, immersion or oral delivery (Komar et al. 2004; Muktar, Tesfaye, and Tesfaye 2016). However, developing fish vaccines that are effective with long-lasting immunity, cost-efficient to produce and license, and easy to administer often proves commercially unviable, especially for helminths (Muktar, Tesfaye, and Tesfaye 2016). Hence, only a few fish vaccines have been developed for major diseases (Harikrishnan et al. 2012; Pasnik, Evans, and Klesius 2005).
As a result, the demand for alternative control strategies has grown, leading to increased research on the potential use of plant extracts as sustainable feed supplements to support health management and reduce the severity of parasite infections when used in preventive mode (Immanuel et al. 2009; Wunderlich et al. 2017). Many studies have highlighted the potential application of phytochemicals such as alkaloids, flavonoids, phenolics, terpenoids and essential oils (EOs) for treating parasitic infections within aquaculture (Anthony, Fyfe, and Smith 2005; Reverter et al. 2014; Tavares-Dias 2018). We previously tested in vitro and in vivo efficacy of herbal compounds using the guppy-Gyrodactylus turnbulli model in bath treatments (Schelkle, Snellgrove, and Cable 2013). Here, we used the same system to assess the effectiveness of the functional feed additive Apex® Branchia, marketed as a health solution to reduce the severity of infections by monogenean gill parasites (Adisseo 2024).
Materials and Methods
Host and parasite origins and maintenance
Wild-type guppies (Poecilia reticulata) originated from the Lower Aripo River, Trinidad, in 2012, but were then maintained in the lab first in Exeter University, and then from 2018 in Cardiff University Aquatics Lab, where they were kept for breeding. Ornamental guppies used in the experiments were purchased from a wholesaler immediately before the start of experiments and were treated once with the anti-bacterial and anti-fungal drug Binox as a precautionary step (Russo and Dillehay 2005). Stocks were housed in 70 L tanks of dechlorinated water (70 fish per tank) in 24 ± 0.5 °C and a 12:12 h light: dark photoperiod. Fish were being fed daily with tropical fish flakes (Aquarian®) supplemented with live Daphnia magna and live freshly hatched Artemia nauplii cultured at Cardiff University Aquatics Lab.
The parasites used for experimental infections were the Gt3 strain of Gyrodactylus turnbulli, which originated from an ornamental stock of guppies purchased in Nottingham in 1997. A culture of parasites was created by isolating a single parasite from an infected ornamental guppy and infecting a naive fish. By infecting multiple fish, a culture was created, which has since been maintained in multiple 1 L containers at 24 ± 1 °C under a 12:12 hr light: dark photoperiod. In brief, each container houses a maximum of four fish, infected collectively with approximately 40 worms. The culture is maintained by screening fish every 72 hr, so any heavily infected fish is replaced with naive, in order to keep the parasite population viable (Stewart et al. 2017). Naive fish came from specific pathogen free cultures, where fish have never been exposed to parasites and no immune response has been initiated.
Experimental method
For this study, a common experimental approach was established to provide consistency across multiple feed trials. Even within the feed trial, treatments were tested in fish of different strains and age, allowing for a greater sample size and consistency of the results. All feed trials were conducted blind, during both experimental procedures and data analyses. Overall, 40 treatments produced by Adisseo were tested here, in one or multiple rounds (Adisseo 2024). Due to confidentiality, specifics about any products or ingredients incorporated into the feeds could not be disclosed, with the exception of one commercial solution Apex® Branchia which we compared to a diet without the functional feed additive (Control). Apex® Branchia is a mixture of organic acids, inactivated yeast and yeast extracts, herbal extracts and essential oils on a mineral carrier that aims to reduce the impact of monogenean gill parasites on productivity and survival of fish, by promoting fish immune competency (Adisseo 2024).
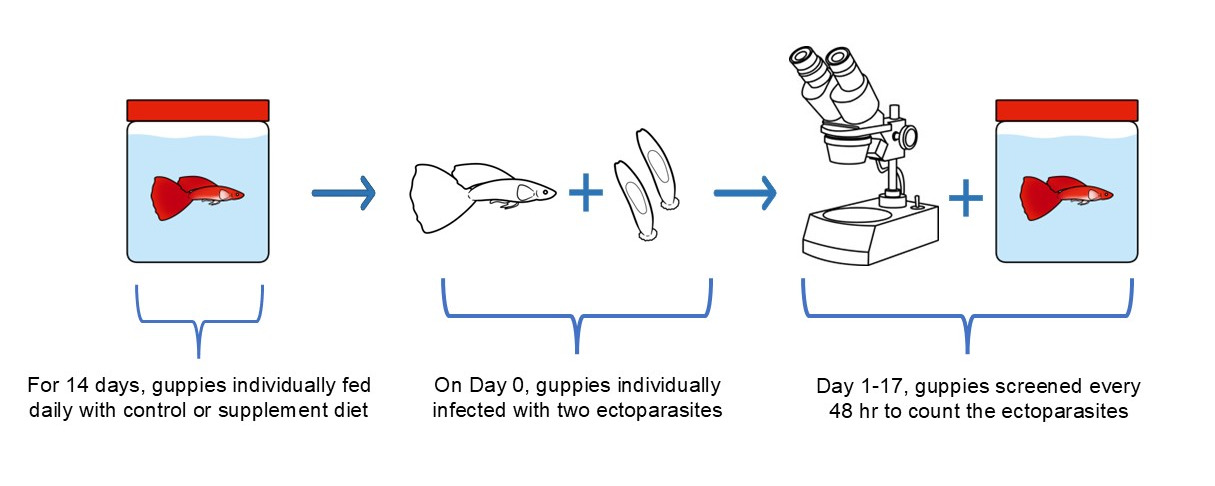
In brief, for each feed trial, fish were anaesthetised with 0.2% tricaine methanesulfonate (MS222), size-matched and placed individually in 1 L pots of dechlorinated water. Once assigned into treatments, fish were fed once daily (15:00 pm) with their respective diet (2% of their body weight; Levy et al. 2015) and their water was changed every 48 hr for 14 days. After two weeks of feeding, fish were anaesthetised and infected with two G. turnbulli worms (Day 0 of infection). For experimental infections, a heavily infected fish, the donor, was overdosed with MS222 and then sacrificed by pithing. By placing the donor close to the anaesthetised recipient, direct contact allowed for transfer of G. turnbulli worms from the caudal fin of donor to that of the recipient fish. All infections and subsequent screening were observed under a dissecting microscope with fibre optic illumination. The recipient fish was returned to its 1 L pot to recover. Fish body and fins were screened again the next day (Day 1 of infection) to ensure the infection was successful and subsequently every 48 hr, so that the parasite number was monitored and recorded for 17 days (Figure 1). During that period, they were still fed daily according to their diet. In the meantime, all feeds were stored in the dark at room temperature when not in use.
Apex® Branchia
The feed trial focused on the efficacy of the commercial solution Apex® Branchia, where fish were provided with a commercial feed formula that is supplemented with Apex® Branchia (0.5%) against the Control (no feed additives), in three different rounds. Round 1 tested Apex® Branchia against the Control diet in naive wild juvenile guppies (n=17 per treatment; mean standard length 8.8 ± 0.6 mm). The efficacy of Apex® Branchia was then tested on naive wild-type (n=18 per treatment, 13.5 ± 0.6 mm) and ornamental (n=14 per treatment; 24.75 ± 4.14 mm) female adult guppies in Round 2 and in Round 3, respectively. After the 17-day trial in Round 2, surviving fish retained in their 1 L containers continued to be fed with their respective diets, and then one month after the first experimental infection, remaining fish (n=10 per treatment) were re-infected (Day 45), to investigate how the treatment, in combination with the host’s immune response, would impact re-infection (Round 4).
Once, experimental trials concluded, infected fish were treated with the anti-helminthic drug Levamisole (0.1%) to eliminate any parasites and were screened clear three consecutive times to ensure that fish were parasite free (Schelkle et al. 2009).
Statistical analysis
For statistical analyses, the parasite metrics examined were mean parasite number, maximum parasite number over the 17-day infection trajectory and parasite count of each fish on each individual day. Using the ‘lme4’ library (Bates et al. 2015), two Generalised Linear Models (GLMs) were constructed to assess which treatment had the best efficacy, therefore treatment was the independent term. For mean parasite number, the models were fitted with a ‘Gamma’ family and ‘logit’ link function, whereas for maximum parasite number, the model was fitted with ‘quassipoisson’ family and ‘identity’ link function. Additionally, two GLMs fitted with a ‘binomial’ family and ‘logit’ link function, were then used to determine whether the survival percentage of the fish and the percentage of fish that cleared their infection were significantly affected by treatment with yes/no responses. Finally, a Generalised Linear Mixed Model (GLMM), was used to examine the parasite count on each fish on each day individually to determine if there was a significant difference in parasite count between treatments. The model included fish ID as a random term to account for repeated measures. As fish for all treatments were size-matched at the start of the feed trails, fish size variability was not taken into account. For all models, error families were chosen based on the lowest AIC values and the robustness of the models was assessed with visual examination of model plots to check standardised residuals, for normal distribution and homogeneity of variance (Bates et al. 2015; Pinheiro and Bates 2000). In all tests, the level of significance was taken as p < 0.05. All statistical analyses were performed in the R statistical software version 4.1.1 (R Core Team 2019). Data is only reported on the most effective treatment.
Results
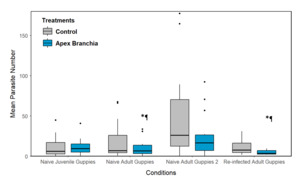
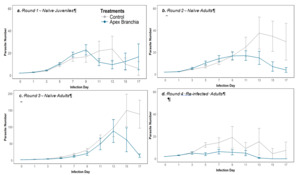
Apex® Branchia had a significantly lower mean parasite number across multiple experimental rounds, indicating the effectiveness of the in-feed supplementation in reducing parasite load (Figure 2). In both naïve and re-infected adult guppies, the maximum parasite number was significantly lower for Apex® Branchia fed fish compared to the controls.
Validation of Apex® Branchia
i. Round 1 - Naive juvenile wild-type guppies
Using naïve juvenile wild-type guppies, fish survival following infection with Gyrodactylus turnbulli, was almost significantly affected by treatment, with treatment Apex® Branchia increasing fish survival compared to the Control (GLM; p = 0.06). Even though mean and maximum parasite number (Figure 3a) as well as number of fish that cleared their infection were not significantly different, fish on Apex® Branchia generally performed better compared to the Control, with lower parasite burden and increased host survival (Table 1).
ii. Round 2 - Naive adult wild-type guppies
In Round 2, fish fed Apex® Branchia had a lower mean (GLM; p = 0.095) and maximum parasite number than the Control (GLM; p < 0.001; Figure 3b). When looking at parasite count across all individuals on each screening day (every 48 hr), treatment Apex® Branchia was also lower than the Control. Even though, fish survival as well as number of fish that cleared their infection were not affected by treatment, percentages were still higher than the Control (Table 1).
iii. Round 3 - Naive adult ornamental guppies
Naive adult ornamental guppies fed Apex® Branchia had lower mean (GLM; p = 0.112) and maximum parasite numbers than the Control (GLM; p < 0.001; Figure 3c) and a lower parasite count over the 17-day infection trajectory, despite a lower survival rate exhibited in ornamental fish (Table 1).
iv. Round 4 – Re-infected adult wild-type guppies
Previously infected adult fish fed Apex® Branchia performed better compared to those fed the Control diet (Table 1). Specifically, the Apex® Branchia group had a lower mean (GLM; p = 0.022), maximum parasite number (GLM; p = 0.018) and parasite count than the Control (GLMM; p = 0.052; Figure 3d; Table 1).
Discussion
The commercial product Apex® Branchia is an effective functional feed additive, promising in reducing the severity of Gyrodactylus turnbulli infection on guppies. It increased survival rate of infected juveniles by at least 30% (Round 1) and reduced parasite load by 40-50% in adult guppies, during primary (Rounds 2 and 3) and secondary infections (Round 4). As illustrated by Schelkle, Snellgrove, and Cable (2013), this host-parasite model provides a reliable and simple testing model for treatments against infectious diseases, generating consistent results across multiple feed trials.
Medicinal plant products have been used as potential therapeutic measures for modulating the immune response and, specifically, the use of herbs to prevent and control fish diseases (Galina et al. 2009; Ji et al. 2012). Efficacy of essential oils is influenced by both time and dose (Soares et al. 2016; Mukherjee et al. 2014; C. F. Williams and Lloyd 2012). Prolonged exposure can improve fish growth rate and food conversion ratio (Talpur and Ikhwanuddin 2012), as well as reduce parasite burden on the host (Abd El-Galil and Aboelhadid 2012). However, there is also potential for overdosing with adverse effects (Yin et al. 2006). For instance, histological examination of fish fed a diet supplemented with 20% garlic powder revealed elevated muscle dystrophy (Fridman, Sinai, and Zilberg 2014). Consequently, dosage optimization is essential to minimize negative outcomes (Galina et al. 2009). Other factors influencing the effectiveness of botanical compounds include the source, geographical origin, strain, age of the product, seasonality, climate and preparation methods, all of which can alter the concentration of active components (Burt 2004; Lawson, Wood, and Hughes 1991). Preparation processes, such as freeze drying and mincing, can significantly alter the effectiveness of products (Lemar, Turner, and Lloyd 2002; Schelkle, Snellgrove, and Cable 2013). For example, the number of parasites was significantly reduced on infected fish exposed to garlic from different sources, treatments with minced and granule forms reduced worm burdens by 66% and 75% after three doses, whereas Chinese freeze-dried garlic and allyl disulphide were 95% effective after a single application (Schelkle, Snellgrove, and Cable 2013). Therefore, botanical compounds need to be adjusted according to the host-parasite system, method of application and nature of the ingredients (Chitmanat, Tongdonmuan, and Nunsong 2005; Schelkle et al. 2009).
In-feed prophylactic supplementation is generally preferable to bath treatments (e.g., Hirazawa et al. 2004; Sutili et al. 2016) as oral administration is non-stressful and non-invasive (Dunn et al. 1990). Even though fish may not consume the entire feed delivered, less product is wasted and released to the environment, minimising any negative environmental impacts (Dunn et al. 1990). In bath applications, natural compounds directly target ectoparasites, potentially affecting their membrane permeability and fluidity, leading to cell death, similar to the mode of action of pesticides and bactericides (Cox et al. 2001; Horn and Duraisingh 2014; Reddy and Kim 2015). In contrast, in-feed essential oils can enhance host immunity over a conditioning period, typically of at least 14 days (Nya, Dawood, and Austin 2010; C. F. Williams and Lloyd 2012). For example, garlic and allicin supplements, in the form of oral treatment, increase production of phagocytic, lysozyme, and anti-protease activities, strengthening the host’s immune system to combat infections (Cristea et al. 2012; Jones 2001; Militz et al. 2013). The current study involving a minimum 14-day in-feed supplementation significantly reduced Gyrodactylus parasite load on guppies and increased host survival, indicating that botanicals can be a strategy to support health management and reduce the severity of parasite infections.
In summary, in-feed supplementation of functional feeds additives formulated to reduce the severity of parasite infections is a sustainable solution to minimising infection (Reverter et al. 2014; Tavares-Dias 2018). Apex® Branchia, a commercial product, is promising against Gyrodactylus ectoparasites, with wider potential use in aquaculture.
Ethical standards
All applicable institutional guidelines for the care and use of animals were followed (Kilkenny et al. 2014). Procedures and protocols were conducted under UK Home Office licence (PPL 303424) with approval by the Cardiff University Animal Ethics Committee.
Acknowledgments and Funding Sources
This research was funded by Adisseo and Knowledge Economy Skills Scholarship (KESSII), supported by European Social Funds (ESF) through the Welsh Government. KESSII is a pan-Wales higher level skills initiative led by Bangor University on behalf of the HE sector in Wales. It is part funded by the Welsh Government’s ESF convergence programme for West Wales and the Valleys.
Conflicts of Interest
WGNO and MMIS are employees of Adisseo.
Author Contribution Statement
EA, JC, WGNO and MMIS conceived and designed the study. EA and JC carried out the experimental feed trials, analysed the data and wrote the manuscript. All authors approved the final manuscript.