1. INTRODUCTION
The Amazon Basin is a significant source of wild-caught ornamental fish for the global aquarium industry (De Sousa et al. 2021). Colombia, Peru, and Brazil are the main suppliers of Amazonian ornamental fish with most traded species belonging to the families Potamotrygonidae, Osteoglossidae, Characidae, Loricariidae, and Callichthyidae (Ortiz and Iannacone 2008). The city of Iquitos, located in the Peruvian Amazon, is the most important region for ornamental fish exports. In 2024, Peruvian ornamental fish exports reached a Free on Board (FOB) value of US$ 4 850 000 and the main destination markets were China, the United States, Germany, Japan, Thailand, Singapore, United Kingdom and Taiwan (PromPerú 2025). Some of the main species of wild-caught ornamental fish from the Peruvian Amazon are “Bleeding-heart Tetra” Hyphessobrycon erythrostigma, “Emerald Catfish” Corydoras splendens, “Marbled Hatchetfish” Carnegiella strigata, “Angelfish” Pterophyllum scalare, and “Xenocara” Ancistrus temminckii, (PNIPA 2021).
Inappropriate management practices such as poor water quality, improper nutrition, and high stocking density as well as poor biosecurity measures in ornamental fish wholesale facilities can cause the emergence of infectious diseases (Cardoso et al. 2019). One of the largest threats to the ornamental fish industry are viruses from the Iridoviridae family, such as Megalocytivirus and Ranavirus, which can lead to high mortality rates and economic losses (Maganha et al. 2018; Sivasankar et al. 2017). According to the Committee on Taxonomy of Viruses (ICTV) Megalocytivirus pagrus 1 is a species within the genus Megalocytivirus (subfamily Alphairidovirinae, family Iridoviridae), which was previously called infectious spleen and kidney necrosis virus (ISKNV), and includes three genotypes: red seabream iridovirus (RSIV), reddish body iridovirus (TRBIV) and ISKNV (Fusianto et al. 2023). Recently, the WOAH Aquatic Animal Health Code has included the Megalocytivirus pagrus 1 in its list of fish diseases (WOAH 2024).
The presence of these viruses has been investigated in several studies involving wild-caught ornamental Amazonian fish. In Brazil, a study reported that 47% of 24 fish species collected from ornamental fish wholesale facilities (including Arapaima gigas, Hypostomus plecostomus, Pterophyllum scalare and Pygocentrus nattereri) tested positive by PCR for the genus Megalocytivirus (Maganha et al. 2018). Also, the genus Megalocytivirus has been detected in Amazonian species including Astronotus ocellatus, Symphysodon sp., Apistogramma cacatuoides and Paracheirodon innesi (Jeong et al. 2008; Nolan et al. 2015; Baoprasertkul and Kaenchan 2019). Furthermore, in Germany, PCR assays confirmed the presence of ISKNV in samples from angel fish Pterophyllum altum associated with mortality events (Jung-Schroers et al. 2016).
Besides, the genus Ranavirus has a wide range of fish hosts and is listed by WOAH (Leiva-Rebollo et al. 2024). Regarding Amazonian fish, a study on the importation of ornamental species into the Europe Union found no conclusive evidence of Ranavirus infection in certain Amazonian species (Vesely et al. 2011). To the best of our knowledge, no virus detection studies have been developed in ornamental fish from the Peruvian Amazon.
The great diversity of ornamental fish species, the scarcity of research on their diseases, and the lack of regulation in the ornamental fish trade industry in most countries may have contributed to the limited implementation of surveillance plans for ornamental fish by authorities. Australia is one of the few countries that has developed a surveillance plan for Megalocytivirus in ornamental fish production facilities (Hood 2021). In recent years, some health authorities are beginning to introduce new requirements for the certification of ornamental fish to prevent the spread of diseases, which makes the early detection and active surveillance of viral pathogens important to prevent and control diseases in ornamental fish (Girisha et al. 2021).
The present study is the first to investigate and survey the presence of the genus Megalocytivirus and Ranavirus in wild-caught Amazonian fish species located in ornamental fish wholesale facilities from Iquitos, Peru, according to the guidelines from the WOAH Aquatic Animal Health Code.
2. MATERIAL AND METHODS
2.1. Fish
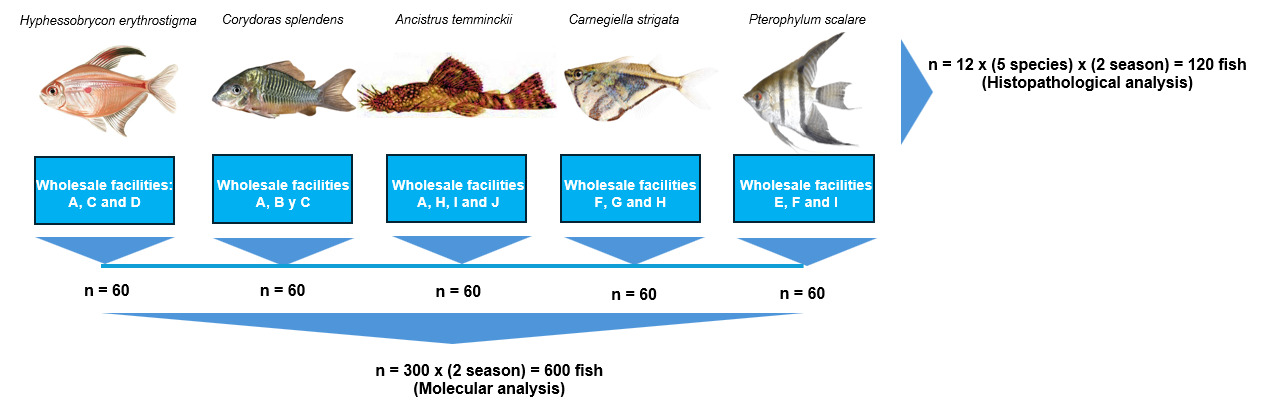
This study focused on five wild-caught Amazonian fish species traded as ornamental fish: “Bleeding-heart Tetra” Hyphessobrycon erythrostigma, “Emerald Catfish” Corydoras splendens, “Marbled Hatchetfish” Carnegiella strigata, “Angelfish” Pterophyllum scalare, and “Xenocara” Ancistrus temminckii. All the fish come from the main rivers of the Amazon Basin and are kept in wholesale facilities located in Iquitos, Peru (Table 1). These facilities are supplied with ground water, and the fish are kept in glass tanks equipped with handmade filters.
2.2. Study design
In 2022, fish from 10 ornamental fish wholesale facilities were sampled in two different periods: one during the dry season (August to September) and the second during the rainy season (June to July). The sample size was determined to provide 95 % confidence in detecting Megalocytivirus and Ranavirus with a design prevalence (minimum expected prevalence) of 5%, according to the WOAH Aquatic Animal Health Code, which resulted in a sample size of 60. So, during the study, 60 fish of each of the five target species were collected from ornamental fish wholesale facilities in each season, resulting in a total of 600 fish for molecular analyses that were tested as pool of five fish. Additionally, during the same visits to ornamental fish wholesale facilities in both seasons, 24 fish from each target species were collected for histopathological analysis, totaling 120 fish. The study design is shown in Figure 1.
2.3. Fish collection
Fish from the five target species showing nonspecific clinical signs (melanosis, fin erosion, hemorrhagic skin lesions) were collected at each wholesale facility after being held for a minimum period of one week. The selected fish was showing unspecific clinical signs as (fin erotion, melanosis, erythema, discoloration). They were placed in plastic bags containing 3 to 5 L of water, filled with oxygen, sealed with rubber bands, and transported to the National Authority for Health and Safety in Fisheries and Aquaculture (SANIPES) facilities, approximately a 30-45 min drive. Upon arrival at SANIPES, all fish were euthanized by overdose with a benzocaine solution (100 mg L1) (Sigma-Aldrich) and immediately examined for gross pathology. Kidneys, spleens, and livers from samples were then removed, pooled, and placed in tubes containing 95 % ethanol for molecular analysis and kept at -20°C until use. Tissues (esophagus, liver, spleen, kidney, gastrointestinal tract and gills) from each fish were immediately fixed in 10% neutral buffered formalin for histopathological analysis.
2.4. PCR detection of Megalocytivirus and Ranavirus
a) DNA Extraction
The ReliaPrep™ gDNA Tissue Miniprep System (Promega, Madison, WI, USA) was used to extract total DNA from 25 mg of pooled tissue (Kidney, spleen and liver), following the manufacturer’s instructions. The purified DNA was quantified using a Qubit 4 fluorometer (Thermo Scientific) before the PCR assays. DNA quality was assessed using an Epoch 2 microplate spectrophotometer (Biotek Instruments) and diluted to 25 ng μL–1. The final product was stored at -20 °C until use.
b) qPCR for Megalocytivirus and Ranavirus
The qPCR protocol involved a reaction mixture of 20 μL, consisting of 10 μL of TaqManTM Universal Master Mix II with UNG (Applied Biosystems), 0.4 μL of Probe (Applied Biosystems), 0.8 μL of each primer (0.4 μM), 2 μL of template DNA, and 6 μL of nuclease-free water. Table 2 shows the primer sets and probes and Table 3 shows the cycling conditions. The analysis was performed using QuantStudioTM Real-Time PCR Instrument and QuantStudioTM and Design and Analysis Software version 1.4 (Applied Biosystems). The synthetic sequence of 125 bp part of the MCP (major capsid protein) gene of the KagYT-96 isolate (GenBank: MK689686.1) inserted into plasmid pCR2.1-TOPO vector RSIV_RT Bam HI (GENSCRIPT-USA) was used as a positive PCR control for Megalocytivirus and the synthetic sequence of 316 bp part of the MCP (major capsid protein) gene of the strain LY-FV3-20161023 (GenBank: MG637360.1) inserted into the pCR2.1-TOPO vector MCP-1_RT_RANAVIRUS was used as a positive PCR control for Ranavirus. Molecular biology grade water was used as a negative PCR control.
2.5. Histopathology
The tissue was fixed in 10% buffered formalin for 24 h. Then, the samples were dehydrated in increasing concentrations of ethanol, diaphanized in xylol and embedded in paraffin wax using HistoCore PEARL (Leyca Byosistems). Finally, samples were sectioned at a thickness of 3.5-4 µm, stained with haematoxylin and eosin (H&E) and examined using light microscopy to determine histopathological alterations. The identification of parasites in tissue sections was performed according to Bruno, Nowak, and Elliott (2006).
3. RESULTS
The assays detected no Megalocytivirus or Ranavirus DNA in any of the ornamental fish samples collected from the ten ornamental fish wholesale facilities in the city of Iquitos during both the rainy and dry seasons of 2022 (Table 4).
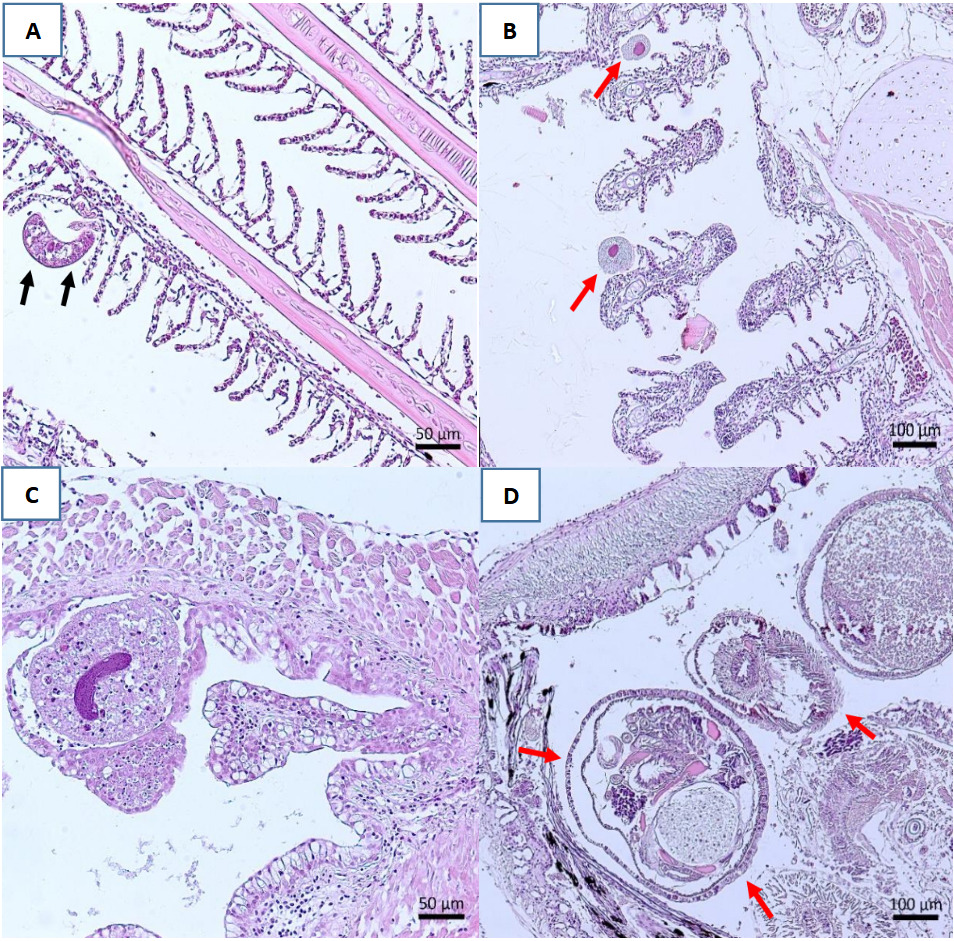
The histopathological examination did not reveal hypertrophied cells and inclusion bodies characteristic of Megalocytivirus-infected fish; however, some parasites were detected (Table 5), including the presence of monogenoids in the gills of C. splendens, P. scalare, C. strigata and A. temminckii. Additionally, Piscinoodinium sp., a dinoflagellate parasite, was found in the gills of C. splendens. Metacercarial cysts of digeneans were observed in the gastrointestinal tract of H. erythrostigma and A. temminckii. Finally, Ichthyophthirius sp. was detected beneath the mucosal epithelium of the esophagus of A. temminckii (Figure 2).
4. DISCUSSION
The current study did not detect DNA of viruses within the genus Megalocytivirus in five species of wild-caught ornamental fish from the Peruvian Amazon. Studies on the presence of Megalocytivirus genus have been reported in Amazonian ornamental fish from Brazil, including P. nattereri (Cardoso et al. 2017), H. plecostomus and P. scalare (Maganha et al. 2018). In addition, this genus has been reported in two Amazonian fish species for consumption, Arapaima gigas and Pseudoplatystoma corruscans (Maganha et al. 2018; Fonseca et al. 2022). To better understand the circulation of this virus in the Peruvian Amazon, further surveys should include a wide range of wild-caught ornamental fish species, based on the fish diversity present in the region and the potential risk of undetected infections. Recently, the technical document “2022 Report of the WOAH ad hoc Group on susceptibility of fish species to infection with WOAH listed diseases” showed which species are susceptible to the three genotypes RSIV, TRBIV and ISKV, including the species P. scalare as susceptible to ISKNV genotype.
Regarding Ranavirus, the five species of wild-caught ornamental fish showed no positive results. A study on the importation of ornamental fish into the Europe Union found no conclusive evidence of Ranavirus genus presence in species such as Carnegiella marthae, C. strigata, Corydoras hastatus, Corydoras julii, H. erythrostigma, H. plecostomus, Monocirrhus polyacanthus, Potamotrygon hystrix and Pseudoplatystoma fasciatum (Vesely et al. 2011).
Pooled sampling was employed in this study in accordance with the WOAH Aquatic Manual, considering that pooling is an effective strategy for reducing analytical costs in surveillance plans (Muniesa et al. 2014). Nevertheless, diagnostic sensitivity may be reduced due to a dilution effect (Laurin et al. 2019) meaning that a larger number of samples is required to demonstrate the absence of Megalocytivirus and Ranavirus.
Although no viral DNA was detected in the current study, the potential risk of disease introduction cannot be ruled out, particularly through the importation of exotic ornamental fish into aquarium facilities (Koda et al. 2023). Supporting this concern, in Australia, ISKNV-like viruses, although the not been detected in wild fish populations, have been identified in imported ornamental fish and has been associated with high mortalities during quarantine (Mohr et al. 2015; Nolan et al. 2015).
The “2023 Report of the Meeting of WOAH Aquatic Animal Health Standards Commission” proposed a new chapter concerning the international movement of ornamental aquatic animals, in response to the potential risk of introducing and spreading fish pathogens. In this context, disease surveillance plans for ornamental fish are particularly important in countries with a significant ornamental fish industry.
Recent studies have employed diagnostic tests for disease surveillance in both wild and captive fish populations (Johnson et al. 2019; Koda et al. 2023). The Australian government has conducted surveillance plans for Megalocytivirus in ornamental fish facilities (Hood 2021) and has developed a risk-based surveillance system for megalocytiviruses, spring viraemia of carp virus and Aeromonas salmonicida in imported ornamental fish (Hood et al. 2019). In Peru, the official surveillance plan for diseases is conducted by SANIPES and focused on farmed aquatic species including whiteleg shrimp (Penaeus vannamei), rainbow trout (Oncorhynchus mykiss) and Tilapia (Oreochromis sp.) (SANIPES 2024). Nevertheless, no targeted surveillance activities are currently conducted for viral diseases in ornamental fish, despite Peru being one of the top exporters of Amazonian ornamental fish. The present study represents a first attempt to assess the presence of viral pathogens in wild-caught fish from the Peruvian Amazon. Although no viral DNA was detected, this baseline data highlights the need for further research focused on disease-susceptible ornamental fish species. Such data is essential to evaluate whether implementing a national surveillance program for ornamental fish is justified.
The study also includes histopathological examination, an important tool for detecting fish diseases. This method is considered as one of the 12-point checklists for designing and applying active disease surveillance in aquatic organisms (Bondad-Reantaso et al. 2021). The histopathological results revealed no lesions typically associated with Megalocytivirus-infected fish, such as enlarged cells and inclusion bodies, as described in previous studies (Jung-Schroers et al. 2016). Also, the histopathological results revealed the presence of monogenoids attached to the secondary gill lamellae of C. splendens, P. scalare and A. temminckii. These findings are consistent with previous histopathological studies on Amazonian ornamental fish, which have reported the presence of gill and skin monogenoids in fish species (Jerônimo et al. 2014; Ramos-Espinoza, Chuquipiondo, and Serrano-Martínez 2017; Dias, Ferreira, and Videira 2021). In heavy infections, monogeneans can damage fish by feeding on mucus and epithelial cells of the skin and gills (Chong 2022). However, the examination of the gills showed a low presence of monogenoids in the fish. According to Jerônimo et al. (2022), monogenoids infestations tend to increase under conditions of high fish density, low water flow, and elevated levels of organic matter.
In addition, the study identified Piscinoodinium sp. trophonts located between the gill filaments of C. splendens. These dinoflagellate parasites have previously been reported in Brazilian ornamental fish, including Corydoras spp. and C. splendens, and are known to cause hypertrophy, hyperplasia and edema in the gills (Ferraz and Sommerville 1998). Piscinoodinium pillulare* was also detected in eight species of ornamental fish farmed in Southern Brazil which it exhibited a higher prevalence compared to other protozoa parasites (Florindo et al. 2017).
This study revealed the presence of Ichthyophthirius sp. embedded beneath the mucosal epithelium of the esophagus of A. temminckii. An experimental study in the catfish Ictalurus punctatus also reported the presence of this protozoan in the peritoneal cavity (Maki, Brown, and Dickerson 2001). These locations are unusual since I. multifiliis is typically found on mucosal surfaces such as skin and gills (Matthews 2005). Among eight ornamental fish species from the Brazilian Amazon, C. strigata, C. martae and P. scalare showed the highest infection rates due to I. multifiliis (Tavares-Dias, Lemos, and Martins 2010).
The study found metacercariae and adult digeneans in the gastrointestinal tract of A. temminckii fish. Digeneans are parasites that require multiple hosts to complete their life cycle, involving mollusks as the first intermediate host, fish as the second intermediate host, and piscivorous birds as the definitive host (Hoshino, Hoshino, and Tavares-Dias 2018). As a result, most digeneans species cannot complete their life cycle within aquarium facilities due to the absence of intermediate hosts. Previous studies have reported the presence of several species of digeneans in Peruvian ornamental fish, including Potamotrygon motoro (Ramos-Espinoza, Chuquipiondo, and Serrano-Martínez 2017), Oxidoras niger (Pantoja et al. 2018) and Brochis multiradiatus (Cuadros et al. 2018). Migration of metacercariae can cause massive tissue erosion with inflammation and necrosis at the site of infection (Williams, Hernandez-Jover, and Shamsi 2023).
Overall, the Amazonian ornamental fish supply chain involves fishermen, intermediaries, and exporters. Throughout these stages fish are exposed to stressors such as handling, overcrowding, and poor water quality, which can lead to mortality and immunosuppression, increasing susceptibility to pathogens (Larcombe et al. 2025).
Therefore, it is necessary for ornamental fish wholesale facilities to implement biosecurity measures to prevent the introduction and spread of pathogens, especially in those facilities that export fish and are required to meet the aquatic animal heath requirements of importing countries.
In conclusion, this study showed that Megalocytivirus and Ranavirus were not detected in at least five wild-caught fish species from ornamental fish wholesale facilities. In addition, this study represents a preliminary survey of ornamental fish species from the Peruvian Amazon aimed at detecting viral pathogens known to cause diseases in ornamental fish worldwide. However, further research is needed to detect the occurrence of emerging, and WOAH-listed diseases in ornamental fish, as well as to develop strategies for designing surveillance plans in these species considering the great variety of Amazonian fish species.
Funding
This work was supported by the Programa Nacional de Innovación en Pesca y Acuicultura (PNIPA), Peru.
Competing interest
The authors declare that there are no conflicts of interest.