1. Introduction
Hyperostosis in fish is referred to excessive growth and swelling of bone tissue in a particular part in the skeleton and usually accompanied with periosteal ossification (Gualdie and Czochanska 1990; Smith-Vaniz and Carpenter 2007; Giarratana et al. 2012). It is not a new lesion and has been recorded in many fish species, especially in marine fishes (Grabda 1982; Smith-Vaniz, Kaufman, and Glowacki 1995a; Jawad 2013). In some of the earlier studies it has been suggested that pollution, diseases and possibly evidence of genetic predisposition may be the cause of hyperostosis (McGrouther 1994; Smith-Vaniz, Kaufman, and Glowacki 1995b). Such studies noted that the condition doesn’t effect the edibility of the fish; on the other hand, Meunier, Gaudant, and Bonelli (2010) studied the histological features of hyperostosis in Lepidopus albyi and found that the hyperostosis constituted spongy bone characteristics. From a pathological point of view, they described the lesion as an increase of the periosteal osteogenesis combined with resorption that creates many vascular cavities. However, many hypotheses may explain the cause of this hyperostotic phenomenon but they remain hypothetical; genetic factors seem the more accepted cause in the earlier studies. Thus, the causes and consequences of hyperostosis remain unclear.
In our study, the prevalence, gross lesions and histopathological examination of hyperostosis were studied in two fish species, Scomberoides lysan (native Arabic name: Dalaa) and Pomacanthus sextriatus (native Arabic name: Anfoos) collected from fish markets during routine inspection of filleting processes at El- Jubail province, Saudi Arabia. The aim of this study was to examine the pathological changes of hyperostosis in these two fish species.
2. Materials and methods
2.1. Fish collection
During routine veterinary inspections at fish markets during the filleting process in Jubail province (‘27 ° 57.9’’ N and ‘49° 40’ 43.4’’ E), 2500 bone skeletons of Scomberoides lysan (Dalaa fish) and 250 of Pomacanthus sextriatus (Anfoos fish) were grossly examined between January 2012 to December 2018.
2.2. Pathological examination
2.2.1. Gross examination
All fish skeletons were examined for the presence of bone swelling in spines and/or vertebrae. The prevalence rate of affected fish were recorded and representative samples examined radiographically.
2.2.2. Histopathological examination
For histopathological studies, tissue sections were prepared according to the established method mentioned by Roberts, Smail, and Munro (2012); fresh bone specimens from the affected fish were fixed immediately in 10% neutral buffered formalin, decalcified in formic acid and embedded in paraffin. Five μm thick sections were obtained and then stained with the routine Hematoxylin and Eosin dye, and other necessary histochemical stains (Alizaren red and Perl’s Prussian blue). The lesions were evaluated and photographed under a light microscope (Olympus BX50).
3. Results
3.1. Pathological results
3.1.1. Gross findings
The examined S. lysan were between 75cm to 120 cm total length and weighed between 1.5 kg to 4.5 kg. The hyperosteoid lesion in these fish appeared as multifocal, very hard, round and smooth pedunculated bony swellings in both neural and haemal spines attached to the base of the vertebral column (Figures 1, 2 and 3). In radiographic findings, the intensity of bony tissue varied where the hyperosteoid swelling in the haemal spine appeared smaller and with lower intensity than the bone tissue while those in neural spines were larger and with more condensed calcification (Figure 4). The diameter of the swellings ranged from 1.5- 2.0 cm in diameter; the cut surface appeared rounded and showed hard osseous tissue in the periphery covered with fibrous capsule containing greasy hard white granules in its centers (Figure 5). The granulated greasy central contents seem to comprise of fat necrosis with calcification. The number of the hyperosteoid swellings ranged from 5/ fish (in small sized fish) to 30 (in larger sized fish). In all fishes, the hyperostotic lesion appeared in its mature hard ossified tissue state without evidence of staging of the lesion.
The examined P. sextriatus were 25 to 40 cm total length and 0.5 to 4kg weight. The hyperosteoid swellings in these fish appeared very similar as that observed in S. lysan, but with smaller diameters; found mainly in the neural spines without attachment to the vertebrae (Figures 6 and 7).
3.1.2. Histopathological findings
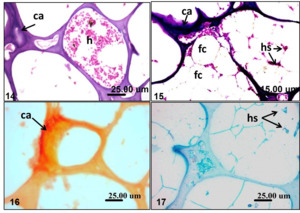
Histopathological sections of hyperosteoid mass in the examined fishes showed prominent acellular bony tissue formed of an external layer of periosteal tissue with a characteristic thin layer of fibroblast cells forming a capsule followed by a thick layer of osteoid tissue with areas of empty spaces underneath (Figure 8). The fibrous capsule showed congested blood vessels and dilated lymph vessels surrounded with edema and the hyperosteoid tissue penetrated by the periosteal tissue (Figure 9). Focal areas of hemorrhages were noticed in the capsular layer (Figure 10). The hyperosteoid tissue appeared with prominent blue colored calcified areas with interlacing empty vacuolar spaces (Figure 11). Areas of hemorrhages were sometimes observed in between the calcified hyperosteoid tissue (Figure 12). The empty spaces were variable in size. The characteristic honeycomb appearance was the common finding (Figure 13). Some of these spaces sometimes filled with hemorrhage together with hemosiderin pigments deposition (Figure 14); while others were impacted with adipocytes (fat cells). The fat cells appeared shadowy and without prominent nuclei indicating fat necrosis. The cells in such areas were surrounded with hemorrhages and hemosiderin pigments together with minute congested capillaries. Prominent deep blue calcification was also noticed (Figure 15). The calcification and hemosiderin pigments deposition was confirmed histochemically using Alizaren red and Perl’s Prussian blue stains respectively (Figure 16 and 17).
4. Discussion
In comparison to mammals, most of the bone tissue of teleosts is entirely devoid of osteocytes (Meunier 1987, 1989; Huysseune 2000; Witten et al. 2004; Shahar and Dean 2013; Davesne et al. 2019). In our study, the bone swelling (hyperostosis) in the two examined fish species Scomberoides lysan and Pomacanthus sextriatus showed multiple acellular bone overgrowth without prominent osteoblast cell proliferation; thus excluding osteoma formation. Hyperostosis in fish is a common lesion and it was recorded in 92 species belonging to 22 families and usually has a species specific pattern (Smith-Vaniz, Kaufman, and Glowacki 1995b). Many hypotheses may explain fish hyperostosis due to pollution, disease problems or genetic factors (Meunier, Gaudant, and Bonelli 2010), but the records of such lesions in many fish families and species exclude the assumption of genetic predisposition. Thus, the actual cause is still unclear. Our histopathological findings were somewhat consistent with the findings of Meunier, Gaudant, and Bonelli (2010); who described the pathological lesions of hyperostosis as spongy bone, increased periosteal osteogenesis combined to resorption that creates many vascular cavities. The examination of hyperostotic swelling in this study added noted some other findings including the aggregation of fat cells in some of the bone spaces in the center of the hyperosteoid tissue. In this regard, the role of the fish spines as a fat reservoir in some fish species has been fully discussed for many years. Wilson (1939) detailed the role of the fish skeleton as a fat depot particularly in the spongy part of the vertebrae and their hollows are frequently heavily laden with fat, and so are the grooves and canals of the neural and haemal spines. The adipose tissue is often present around the bones, however, due to the difficulty of sectioning this region, it has not been examined in histological detail.
In our study, the presence of fat cells in the cavities of the hyperostotic swelling together with the white granular contents in the center of such lesions may indicate evidence of abnormal fat metabolism which might occur as a result of some physiological disturbances during spawning, migration or changes in the nutritional behavior of these two fish species. However, Sutharshin, Sivashanth, and Thulasitha (2013) have paid attention to lipid changes in relation to maturation and spawning of S. lysan, the same fish that showed hyperostosis in our study. Also, Boglione et al. (2013) have demonstrated that different nutrients including lipids are responsible for the appearance of skeletal anomalies. Recently, Hemingway and Scarnecchia (2017) have confirmed the role of life history differences, such as growing season in the lipid metabolism in different tissues of paddlefish Polyodon spathula.
5. Conclusion
The present study concluded that Scomberoides lysan and Pomacanthus sextriatus have a high prevalence of hyperostosis in the El- Jubail province of Saudi Arabia. The pathological lesions of hyperostosis in these two fish species are typically characteristic. Further epidemiological studies are required to make correlations between hyperostosis and other physiological factors such as migration, maturation and spawning. Also, genetic predisposition cannot be neglected.