Introduction
In the current situation of constantly decreasing biodiversity and shrinking world fishery resources, with fish stocks in a state of deterioration, aquaculture represents a reliable source of increasing fish reserves and is one way of ensuring a sufficient amount of fish for food in the future.
The economic importance of rainbow trout (Oncorhynchus mykiss) in the global aquaculture industry, makes it one of the most leading species for inland water fish farming. Because of its rapid growth, and rich and valuable meat composition, rainbow trout is a preferred species for human consumption. Today in Serbia, more than half of consumed fish from total domestic resources are obtained from aquaculture. Rainbow trout represents an increasingly popular species of cultured fish in the Serbian aquaculture industry (Marković and Poleksić 2013). It is reared in different production systems and specially designed facilities (Stoyanova et al. 2016). Although rainbow trout are mostly farmed in cages and classic outdoor raceways, due to the need to protect farmed fish from disease transmission and the need for fresh fish, this species is increasingly farmed in recirculation aquaculture systems (RAS). This system rears fish at high densities, in indoor tanks with an optimised and controlled environment, with recirculating systems which filter and clean the water for recycling back through fish culture tanks.
When reared in high-density indoor systems, fish must be given a high-quality, nutritionally complete, and balanced diet (Craig and Helfrich 2009). The use of feed lacking or containing an excess of components can lead to various physiological disorders in fish (Oliva-Teles 2012). Rainbow trout is carnivorous and needs a diet rich in protein. Because protein is the most expensive component of fish feed, higher percentages of lipids are used in fish feeds. This can lead to the accumulation of unnecessary fat in the liver, thereby threatening the health of the fish and meat quality (Craig and Helfrich 2009). Generally, fish tissues are rich with n-3 polyunsaturated fatty acids (PUFAs), a property that has potential benefits for human health (Ruxton et al. 2005), but the positive nutritional value of long-chain n-3 PUFAs derived from fish oils and meals can become a negative factor for fish (Hsieh and Kinsella 1989). Fish are potentially at risk of peroxidative attack because of the presence of large quantities of PUFAs in both fish tissues and diets (Oliva-Teles 2012). Polyunsaturated fatty acids are susceptible to oxidation because oxygen impacts double bonds, creating lipoperoxides, which produce reactive oxygen species (ROS), and finally induce oxidative stress (Jin et al. 2017). The liver is a target for metabolism in the fish body, and liver biomarkers are useful as indicators of the general health of fish and are considered to reflect physiological exposure to a variety of redox factors (Sadekarpawar and Parikh 2013).
Harmful effects of oxidative stress on the lipids of cellular membranes are neutralised by effective antioxidant defense systems operating in fish (Martínez-Álvarez, Morales, and Sanz 2005). The antioxidant enzyme system includes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and the enzyme of phase II biotransformation glutathione-S-transferase (GST). These enzymes, besides scavenging ROS, are good biomarkers of oxidative stress, and are useful diagnostic and prognostic tools for assessing the effects of a large number of individual and interactive processes in aquatic organisms (Ross et al. 2001).
To minimize production expenses in fish farming, potential threats to fish health need to be taken into account. The present study aimed to provide data about the possible effects of two commercial diets with different compositions on the levels of antioxidant enzymes (SOD, CAT, GPx, GR, and GST) in tissues of aquaculture rainbow trout.
Material and methods
Ethical statement
All experimental procedures complied with the European Council Directive 86/609/EEC for the protection of animals used for experimental and other scientific purposes, the Serbian Animal Welfare Law (‘‘Official Gazette of the Republic of Serbia’’, No 41/09) and Regulations on the Welfare of Animals Intended for Experimental Purposes (‘‘Official Gazette of the Republic of Serbia’’, No 39/10).
Rearing conditions
Rainbow trout from the “Little Danube” Centre for Fisheries and Applied Hydrobiology (the University of Belgrade - Faculty of Agriculture, Serbia) were divided into two aquaculture groups, - AG1 and AG2. Each group was placed in a separate pool and maintained in small indoor recirculating aquaculture systems (RAS) with all conditions needed for effective trout production. Fish were stocked at a density of 200 individuals per m−3 (n ≈ 80 fish per tank). Water temperature (T), pH, dissolved oxygen (DO), and conductivity (EC) were checked daily with the aid of a MULTI 340i set (WTW, Germany). Physico-chemical parameters were obtained by immersing the corresponding electrodes 0.05 m below the water surface in both pools (Table 1). All fish used in the study were of homogeneous age and size. The fish from AG1 were fed with commercial diet 1, while fish from AG2 were fed with diet 2. Formulas and approximate composition of diets 1 and 2 are presented in Table 2. Fish were fed daily by hand at a feeding rate of 1.2-1.5% of total fish body mass in both fish groups following regulation of the temperature and oxygen level.
Fish sampling
Fifteen healthy rainbow trout were randomly sampled using seine nets from each studied group (AG1 and AG2) and then transferred to a laboratory of Belgrade University’s Faculty of Agriculture. Fish were sacrificed with an overdose of 2-phenoxyethanol. Individual fish body weights (BW), total lengths (TL), and liver weights (LW) were taken and used to determine the hepatosomatic index (HSI) and condition factor (CF) using the following formulae:
Hepatosomatic index (HSI) = ((liver weight (g) / fish weight (g)) × 100
Condition factor (CF) = weight (g) / length (cm)3 x 100.
Sample preparation and measurement of antioxidant enzymes
After death, liver, abdominal muscle, and dorsal muscle samples from the fish were excised aseptically, frozen immediately in liquid nitrogen, and stored at −80°C until further analysis. Before the measurement of antioxidant enzymes, tissue samples were thawed, washed with sterile physiological saline, and then dried with filter paper. Approximately 0.5 g of each tissue sample (n=15) was minced and then homogenised in a homogenisation medium (0.25M sucrose, 0.5mM EDTA, and 10 mM Tris–HCl; pH 7.4) with an IKA-Werk Ultra-Turrax homogeniser (Janke and Kunkel, Staufen, Germany) at 4°C (Rossi, Cecchini, and Dianzani 1983). The resulting homogenates were sonicated for 15 s at 10 kHz on ice to release enzymes (Takada et al. 1982). Sonicates were centrifuged at 100 000×g for 90 min (4°C). After centrifugation, the supernatant was separated and used for the determination of total protein concentration and activity assays. Total protein concentration in all samples was quantified by the Bradford method (Bradford 1976) adapted from Bio-Rad’s Bradford micro-assay, using bovine serum albumin as the standard.
Superoxide dismutase activity was analysed following the method of Misra and Fridovich (1972) based on the autoxidation of adrenaline to adrenochrome. The change in absorbance was monitored at 480 nm. The activity of CAT was quantified by the decomposition of H2O2 at 240 nm according to the method of Claiborne (1985). Glutathione peroxidase activity was determined by following the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) as a substrate with t-butyl hydroperoxide (Tamura, Oshino, and Chance 1982). The activity of GR was detected from the oxidation of NADPH during the reduction of GSSG (Glatzle et al. 1974). The activity of GST towards 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate was evaluated according to the technique described by Habig, Pabst, and Jakoby (1974). All measurements were performed in duplicate to ensure representativeness.
Data analysis and statistical methods
Data were analysed using the SAS statistical package and the Microsoft Excel data analysis tool package. Each column on the graph represents the mean of 15 fish ± the standard error of the means (SE). The data were first tested for normality (Kolmogorov–Smirnov normality test) and homogeneity of variance (Levene’s test). One-way analysis of variance (ANOVA) was used to compare means in the two different aquaculture groups. Following analysis of variance, significant between-group differences were detected by Tukey’s posthoc test. Differences were considered significant at P < 0.05. The data presented in the figures for each biomarker inside each group were pooled and correlated with each other. Pearson’s correlation coefficients were calculated for pairs of biomarkers using the Microsoft Excel data analysis tool. Statistical analyses were performed with STATISTICA 8.0.
Results
The higher crude protein and lipid content in diet 2 resulted in an increase in BW and LW in AG2 fish (average weight 135.8 ± 22.1), by ~9 and 11%, respectively, with concerning AG1 fish (average weight 125.30 ± 23.9). The condition factor exhibited no difference between the two groups, while HSI was 7% higher for individuals sampled from AG2 compared to samples from AG1.
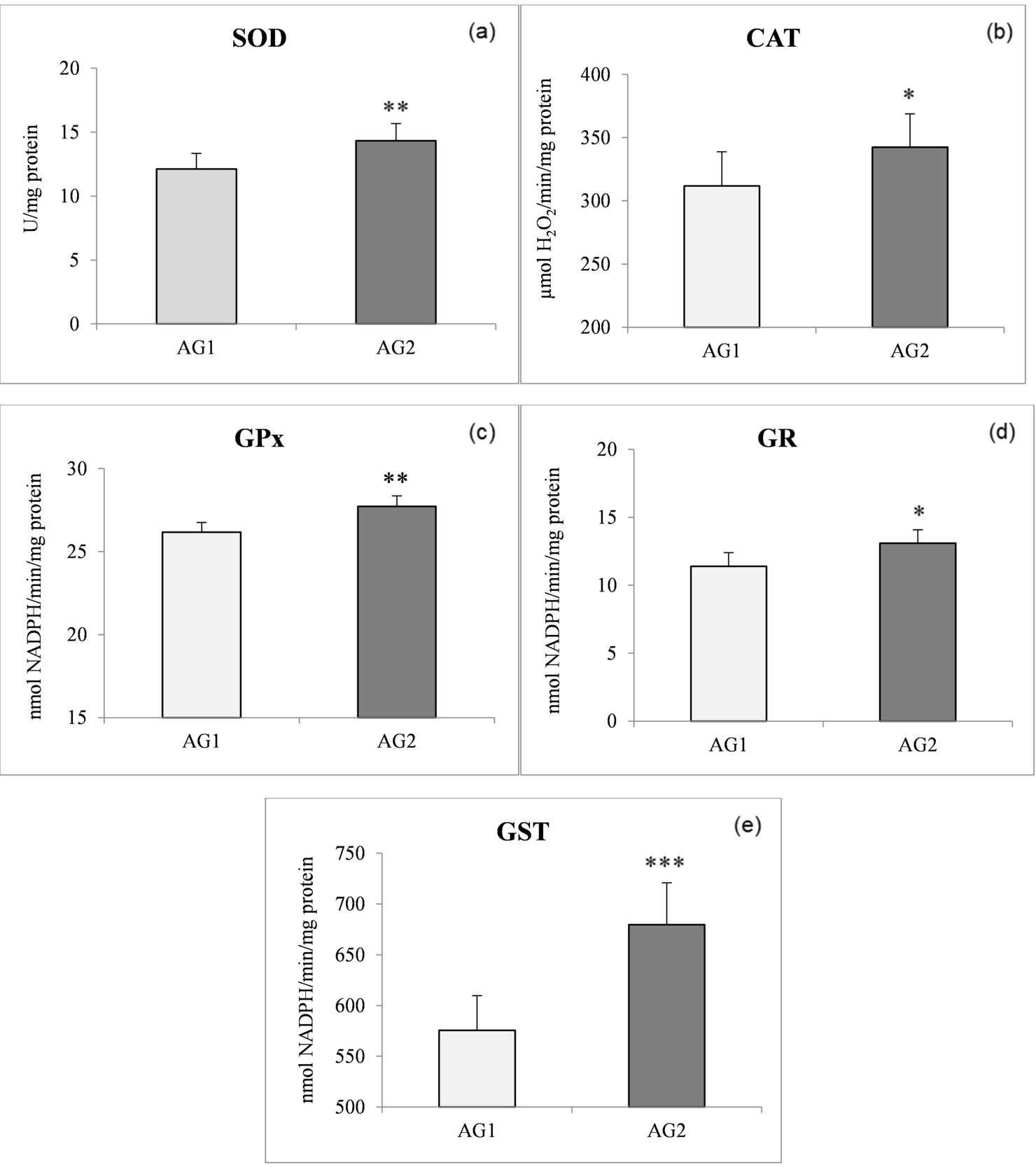
Generally speaking, antioxidant activities in the AG2 fish were higher compared to the AG1 fish. As expected, the highest antioxidant enzyme activities in both populations were found in the liver compared to both muscle tissues analysed. The most pronounced significant difference (P < 0.001) between the two groups was found for liver GST activity (Figure 1e). A significant increase in the SOD (P < 0.01) and GST (P < 0.001) (Figure 1a, e) levels of liver tissue (both by 15%) was found in AG2 compared to the AG1 group. The activities of liver CAT, GPx, and GR in liver tissue displayed significant increases of 9, 5, and 13%, respectively, in the AG2 group concerning AG1 (Figure 1b,c,d). In the AG1 fish, liver SOD activity was positively correlated with GPx activity and GPx was positively correlated with GR, while in fish from AG2, on the other hand, GST activity was positively correlated with SOD, GPx, and GR (Table 3).
The levels of GR and GST in the two types of muscle tissues of trout did not differ significantly in individuals of the same group (Figure 2c), in contrast to the case of GPx activity in individuals from AG2 (Figure 2a), where abdominal muscle activity was significantly higher compared to dorsal muscle activity of the same individuals.
Regarding muscle tissue, Pearson correlations showed positive correlations between GSH-dependent enzymes in the dorsal muscle of the AG1 group, while a positive correlation was noted between SOD and GR activities in the dorsal muscle of the AG2 group (Table 4A). Activities of antioxidant enzymes in the abdominal muscle of both fish groups followed almost the same pattern as that shown for dorsal muscle in Table 4B.
Discussion
Values of body weight, LW, and HIS were higher in the group (AG2) fed with the diet having higher fat content (diet 2) than in the AG1 fish, as was expected (Table 3). In line with the study of Librán-Pérez et al. (2015) on rainbow trout, our results showed that a diet 2 compared to a diet 1 improved fish body weight. Since a high level of HSI is associated with high energy reserves and metabolic activity, these results indicate that fish fed a diet with a higher content of crude lipids had a good nutritional status, but there are several drawbacks to increasing the lipid concentration in a fish feed (Sinclair 2000). Both lipid intake and protein intake cause an acute increase in ROS generation, which indicates that nutrient intake is probably a major modulator of ROS generation (Mohanty et al. 2002). This finding raises the issue of the relative effect of different diets on ROS generation, ROS load, and potential oxidative damage.
Numerous studies have already shown an increase in the activity of antioxidant enzymes when fish were fed oxidised diets (Trenzado et al. 2006; Fontagné et al. 2008) and other papers have discussed the possibility of a PUFA-elicited antioxidant enzyme response in animals (Garrido et al. 1989; Nowak 2013). We assume that the higher % of lipids in diet 2 given to the AG2 group caused an excessive release of superoxide anion radicals, which can cause a fairly intense activation of SOD and CAT. It has been previously shown that induced increases in CAT and SOD activity result both in a reduction of ROS and in oxidative damage (Wilhelm-Filho, Giulivi, and Boveris 1993). Alternatively, the relatively high activity of CAT in the liver (15-20 times higher than SOD activity) in the AG2 population might suggest the existence of permanent protection against the cytotoxic action of H2O2. The low GPx activities recorded in the present study in the liver of both fish populations were linked with extremely high activities of CAT in this organ (Figure 2b, c). This reflects competition between CAT and GPx for the same reactive oxygen species, i.e., H2O2.
Concerning glutathione-related enzymes, it seems that diet 2 evokes an increase of these enzymes in both the liver and the muscles of O. mykiss. As far as GPx activity is concerned, it was significantly weaker than the activity of CAT, but an increase in GPx activity was nevertheless present in the AG2 compared to the AG1fish, which would greatly improve the ability of the fish to detoxify H2O2 or organic peroxides and could promote (via Fenton reactions) lipid peroxidation and oxidative stress (Halliwell and Gutteridge 2006). The activity of GR and GST in both fish tissues also displayed relatively similar increasing trends as shown by GPx activity in the AG2 compared to the AG1 group. Glutathione peroxidase activity is considered complementary to CAT activity, being especially suited for H2O2 detoxification at low substrate concentrations. In addition, GPx reduces a variety of peroxides to their corresponding alcohols with its major detoxification function being to terminate radical chain propagation by quick reduction to yield further radicals (Kono and Fridovich 1982). Glutathione peroxidase is considered the most effective enzyme against lipid peroxidation. In the present study, an increase in the level of GPx activity was recorded in all analysed fish tissues. The existence of a small difference between dorsal muscle GPx activity and abdominal muscle GPx activity in fish fed with different diets indicates a weaker ability of a dorsal muscle to neutralise the impact of peroxides. The results suggest that the higher content of lipids in diet 2 evoked higher GPx activity in the liver of trout from AG2, which follows previous findings in trout and sturgeon (Trenzado et al. 2006).
The activity of GR closely followed that of GPx, as was to be expected (Stoliar and Lushchak 2012). The higher GR activity observed in trout fed diet 2 indicates its active role in pathways of ROS recycling. The induction of GPx and GR activities could be interpreted as a greater demand for reductant power with higher lipid content. Additionally, GSH-associated enzymes such as GR, GPx, and GST cumulatively protect fish against ROS (Srikanth et al. 2013).
Glutathione-S-transferase is highly inducible in animals and humans, and its expression is affected by dietary factors that include lipids (Girón et al. 1999). The present study demonstrated an increase of GPx and GST levels positively correlated with GR activity in all tissues of O. mykiss (Tables 3 and 4). Because the presence of PUFA in the diet is known to increase the levels of peroxides, increased GST activity in fish fed diet 2 compared to fish fed diet 1 could be an indication of a detoxification process aimed at protecting tissues from oxidative damage.
In the liver of fish of the two studied groups, a positive relationship was found between SOD and GPx in the AG1 group and of SOD with GPx and GST in the AG2 group (Table 3), while some findings showed a positive relationship between SOD and CAT in the liver of other fish species (Wilhelm-Filho, Giulivi, and Boveris 1993). This could mean that even minor increases of lipid content in the fish diet could lead to significant physiological changes in O. mykiss.
The findings discussed in the present study suggest that a diet containing more lipids and digestible energy is effective in enhancing enzymatic antioxidant capacity in O. mykiss. Rainbow trout seem to activate antioxidant defense mechanisms in response to increasing amounts of dietary lipids, doing so in a manner efficient enough to prevent serious injury to the tissues. The observed changes probably represent a normal physiological adaptation to different compositions of the two diets used. These data enable us to assert that these changes can have an important role in monitoring the health of fish in conditions of aquaculture. Gaining an understanding of such links should guide the development of stress-based strategies to balance the lipid content of fish diets.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgment
This work was supported by the Serbian Ministry of Education, Science, and Technological Development (contract numbers 451-03-9/2021-14/200007 and 451-03-9/2021-14/200116).