Keynote - Major fish diseases of laboratory fishes: zebrafish and killifish
Olga L. M. Haenen8
Laboratory fish, like zebrafish (Danio rerio) and African turquoise killifish (Nothobranchius furzeri) are worldwide intensively used as model-fish for scientific veterinary and biomedical research and peer reviewed papers on, for instance, genetics, biology, and immunology/diseases. In this keynote lecture, examples of pathogens and disease, and possible consequences of using infected fish in scientific research are presented.
Zebrafish
Over 3,250 institutes in over 100 countries use zebrafish, resulting in the use of over 5 million fish/year. This will only increase, given rapid CRISPR/Cas9 gene editing (Lidster et al. 2017). Costly genetic lines are bred. Zebrafish may suffer various chronic, clinically non- or hardly visible diseases, as they are small fish and need an experienced eye of the fish caretaker. Kent et al. (2012, 2020) described the most important diseases of zebrafish, of which some important ones are summarised in Table 1. Furthermore, zebrafish may be affected by common fish ectoparasites, like Trichodina, Chilodonella, Ichthyophthirius multifiliis, Oodinium, Ichthyobodo, Dactylogyrus, Gyrodactylus, Argulus, etc.. Regarding bacterial infections, opportunistic secondary bacterial infections may occur, when fish are stressed. Furthermore, combinations of parasitic and bacterial infections may commonly occur. Only three viruses have been described so far for zebrafish.
Killifish
The genus Nothobranchius (50 species, Cyprinodontiformes, Nothobranchiidae) is the shortest-living vertebrate bred in captivity, with a lifespan of three to nine months. The African turquoise killifish N. furzeri is ideal for research on aging, and also comprises transgenic strains (Polačik, Blažek, and Reichard 2016; Reichard and Polačik 2019). For this species, only a few diseases are known so far. Di Cicco et al. (2010) described a basic pathology of this species: neoplastic lesions with systemic failure of homeostasis functions, which results in fast aging-related histopathological changes. Apart from that, this species may suffer various parasitic and bacterial infections. Nezhybová et al. (2017) described the parasites of killifish in nature: all were endoparasites, infecting muscles and internal organs. Often also, intermediate parasite stages of birds were detected in wild killifish. Metacercaria larvae of trematodes were detected in the killifish muscle, and a high diversity of flukes (Trematoda), roundworms (Nematoda), and tapeworms (Cestoda) in muscle, intestine, cerebral and abdominal cavities, gallbladder, and gills were described. Moreover, larvae of the fluke Apatemon sp. may infect the cerebral cavity and change the behaviour of killifish (Nezhybová et al. 2017). In fish experimental laboratories, the parasites Amyloodinium, Piscinoodinium pillulare (velvet disease), Oodinium, and Glugea (microsporidean), were found in killifish, among other parasites (Polačik, Blažek, and Reichard 2016). It was advised to use medium hard, lightly basic water to prevent for Piscinoodinium (Schäfer 2009). Regarding bacterial infections, various opportunistic infections were known, like with Aeromonas spp. and Mycobacterium marinum. No viral infections have been described so far for killifish.
Many asymptomatic diseases
The above information illustrates, the ‘ideal’ model-fish species for scientific veterinary and biomedical research may suffer chronic, asymptomatic infections and disease. This means, the outcome of infection trials with infected laboratory fish then will be biased systematically, and as a result the quality of related peer reviewed papers will be less, without realizing. Some of those results are applied in human medicine and drug prescriptions, and therefore may indirectly harm humans, through safety tests of drugs with non-reliable model-fish.
What could we do?
We should take zebrafish and killifish diagnostics and health monitoring more seriously during fish disease diagnostics. We should get skilled in diseases of these species, and pro-actively contact experimental animal facility centres using these fish to educate professionals (caretakers, aquatic veterinarians), and advise them on regular health monitoring. We should train them in prevention of disease, such as not to feed killifish or zebrafish with infected frozen or live feed, as this may cause transfer of fish pathogens, to start with specific pathogen free (SPF) laboratory fish, and to have quarantine facilities and a preventive monitoring for fish disease in place during laboratory fish rearing and experiments. We should practice or develop diagnostic tests for these fish species.
Conclusions
Laboratory fishes are widely used all over the world in science. The fish may suffer various asymptomatic infections and diseases, which may influence the quality and outcome of animal and human science. Laboratory facilities must have skilled fish caretakers supported by aquatic veterinarians, who practice disease prevention, quarantine, and regular health monitoring. EAFP-members can provide expertise on diagnostics and should take actions in cooperation with the other relevant organizations.
Q & A
The lack and the need of skilled fish health experts (mainly in lab fish species) involved in zebrafish research (at facility control level, but also involved in the research assessment) was stressed by F. Padrós. He suggested to organize courses promoted by EAFP; this was indeed underlined as urgent by O. Haenen. - E. Lewisch asked, which method is the most suitable to differentiate between Mycobacteria species. O. Haenen replied, WBVR uses more than two PCR protocols to distinguish the group in which M. marinum belongs from other mycobacteria. - C. Whipps added that they have specific tests for M. marinum and M. haemophilum, being the most important mycobacteria. Most other species are not fish pathogenic. One can culture the bacteria, but this may take weeks to two months, until being able to declare the isolation negative.
Mycobacteriosis in laboratory fishes: a model system using fluorescent Mycobacterium chelonae and transparent zebrafish
C. M. Whipps2 and A. J. Janik
Mycobacteriosis is a common disease in laboratory zebrafish, caused by several different species of Mycobacterium, with infections ranging from severe to subclinical, and acute to chronic (Whipps and Kent 2020). Mortalities in fish stocks can hamper research and underlying infections may contribute to unanticipated experimental variation. Thus, understanding and quantifying the impacts of infection and evaluating methods for control of mycobacteria is a priority for zebrafish health research. In larval zebrafish, pathogenesis of mycobacterial infections have been studied to learn more about vertebrate immune responses (Tobin and Ramakrishnan 2008). One advantage of using larval fish is that they are mostly transparent allowing for real-time visualisation of an infection using a fluorescently labelled bacterium. Evaluating infections in adult zebrafish cannot typically be done in this same manner due to pigmentation in the body wall of the fish. Consequently, such studies have relied on mortality and histology as end points (e.g., Watral and Kent 2007; Whipps, ST, and Kent 2007; Ostland et al. 2008; Chang, KM, and Whipps 2017). A potential path around this obstacle is to use the mutant “Casper” line of zebrafish which lacks melanocytes and iridophores (White et al. 2008), making the body wall transparent and internal organs visible. Mycobacterium chelonae is commonly encountered in zebrafish (Whipps, Matthews, and Kent 2008). While M. haemophilum or M. marinum are more likely to be associated with severe disease patterns in zebrafish (Whipps, C, and Wagner 2012), M. chelonae cause chronic, subclinical infections, and more likely to be overlooked as a source of experimental variation. Using a strain of M. chelonae (H1E2-GFP) labelled with green fluorescent protein (GFP), we have undertaken studies infecting “Casper” zebrafish, permitting the visualisation of infection in anaesthetised fish over time. Using this system, we determined that zebrafish can acquire infections from a biofilm containing M. chelonae in as little as two weeks and that bacteria shed from these fish can be detected in biofilm or faeces samples in one week (Chang, Benedict, and Whipps 2019). We also investigated the potential of transmission via live feeds. The GFP allowed to visualise uptake into paramecium, brine shrimp, and rotifers, and easily observe the resulting infections in zebrafish (Chang, Lewis, and Whipps 2019). In a recently completed study, we evaluated antibiotic treatment in infected “Casper” fish (Figure 1) using tigecycline and clarithromycin, monitoring fish weekly over a 40-day treatment (Janik 2021). A decrease in M. chelonae GFP fluorescence was observed in some individual fish over the course of treatment, but treatment groups did not show clear decreases in GFP over time. We did observe reduction of infection severity by histology in some of the treated groups, which is consistent with our prior studies using only histology as an endpoint (Chang, KM, and Whipps 2017). Results suggest that antibiotic treatment can reduce bacterial burden in zebrafish, but not completely eliminate infections after 40 days. Treatment may be useful in valuable lines of fish to reduce infection severity and return fish to reproductive health. Fish could be treated, bred, eggs surface disinfected, and a new stock established. The lessons learned using “Casper” zebrafish and a GFP M. chelonae are allowing us to investigate aspects of mycobacterial disease, transmission, and control that were previously not possible. Insights from this model have utility beyond laboratory zebrafish, including those working on other aquatic models, or in aquaculture.
Q & A
O. Haenen asked if the method above could also be applied to pathogens of warm-blooded animals and even to humans, and this was thought possible. - E. Lewisch asked about Whipps’ opinion on regular disinfection methods of the stocked system with peracetic acid formulations; he answered that this might work. - F. Padrós asked, if biofilms are also so relevant in the epidemiology of bacterial disease in zebrafish tanks, and how often should tanks be changed and cleaned? C. Whipps answered that mycobacteria will long survive in biofilms in tanks, and best refresh every 2-4 weeks, and space out, to minimize risk. - E. Lewisch asked whether biofilm/faecal samples could replace samples of live fish? – B. Gorgoglione asked, if the biofilm is tested negative, what is the sensitivity of this test when compared to that on fish? Followed a discussion on the accuracy of alternative and non-invasive testing methods. Since M. marinum and M. haemophilum are residing in fish, and not in the biofilm, this alternative sampling is not advised.
From catcher to keeper: the complex trade in ornamental aquatic animals
Nicholas Stinton3
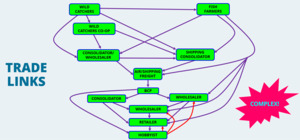
Ornamental fish are extremely popular, being the third most popular pets behind dogs and cats. In the UK it is estimated that there are 2,000 pet shops, employing some 12,000 people, with a total industry value of £400 million (OATA, 2021). It is a truly global industry with animals sourced and shipped across the world with almost every continent involved in the trade. There are regions that are traditionally leaders in supply of such animals such as Africa, Caribbean, China, Europe, Japan, North and South America, Southeast Asia, and Polynesia. Within these regions there are ‘Hubs’ of distribution such as the Czech Republic, Florida, Hong Kong, Singapore. Although the animals supplied are not necessarily produced in those areas. The main markets for these animals are in Europe, Japan, and North America with a growing interest in China (traditionally known for fishkeeping). Animals are sourced from both the wild and are also cultured, most marine species are obtained from the wild (90%) and most freshwater species are cultured (95%) (OATA, 2021). Within the marine environment a wide range of operations from large business to catcher’s cooperatives (fair-trade type initiatives) to individuals/families. A variety of methods are used to catch or collect the species and although there are concerns over wild caught species, the practice is often important in local economics. That said there are increasing efforts to culture species artificially, such as corals which also have additional sustainable benefits, including with the creation of artificial reef ecosystems. Although there are freshwater species caught in the wild, most freshwater species traded are cultured. These are cultured in a wide variety of intensities; from very low tech back-yard operations to extensive aquaculture farms that produce multiple species in intensive recirculation systems. Often operations may specialise in a single species, such as koi carp in Japan. Some operations do rely on wild ‘seed’ which is in turn are totally reliant on the health and status of the ‘seed’ in terms of their success, whereas other operations operate a fully closed cycle in terms of all life stages of production. There are multiple stages involved in the movement of the animals from source countries to end destination and given the global trade most animals are shipped by air (Figure 2). Supplying companies take the form of traditional wholesale set-ups with multiple facilities to hold livestock before shipping or consolidator companies, handling animals already packaged by their suppliers as logistic operations. Shipments will usually pass through a clearance process into a receiving country whereby health status guarantees are checked and import customs duties are collected. Like supplying companies, receiving companies can take the form of wholesale set-ups to consolidator companies in the onwards supply to retailers and ultimately the hobbyist, often with multiple companies involved in the supply chain. Finally, even the hobbyist supplies aquatic animals into the trade, all be it in small quantities.
Q & A
B. Gorgoglione asked Stinton’s point of view, as a veterinarian involved in the ornamental fish sector, whether young veterinarians receive enough and adequate education and practical training at university level, so if they could play a role in the complex trade sector for ornamental aquatic animals. N. Stinton replied that education and training programs are currently not enough, thus there is an urgent need for improvements.
Transboundary spreading of fish viral diseases through ornamental fish trade and release
T. Ito4, O. L. M. Haenen8, Y. Kawato4, T. Mekata4
Freshwater and marine ornamental fishes are traded in more than 125 countries with a total retail value of greater than 10 billion USD annually (Dey 2016). Some of these fishes may carry pathogenic fish viruses, which may cause devastating fish mortalities in importing countries (Ito, Kurita, and Haenen 2017; Haenen et al. 2020). Pathogens and diseases of ornamental fish can easily spread. For example, cohabitations of various batches of imported ornamental fish may cause cross infections in pond fish during their distribution process. Cyprinid herpesvirus 2 (CyHV-2), aka goldfish herpesvirus, has caused devastating economic losses to goldfish culture and trade. Since a CyHV-2 infection of goldfish mostly does not expose specific clinical signs in goldfish (Ito et al. 2013), farmers can unintentionally sell and export these CyHV-2-infected fish all over the world. Such asymptomatic virus-infected goldfish carry a substantial risk to fish populations abroad, when exported. We evaluated the prevalence of CyHV-2 in goldfish imported into the Netherlands. CyHV-2 DNA was detected from four of eight imported goldfish batches tested in 2014 and 2015, most of which were apparently healthy. In addition, the virus was isolated from one of the apparently healthy goldfish batches using a cell culture method. The data strongly indicated that international trade is facilitating the spread of CyHV-2 (Ito, Kurita, and Haenen 2017). In koi/carp aquaculture, koi sleepy disease caused by carp edema virus (CEV) has been considered as one of the concerning diseases (Haenen et al. 2016). It was speculated that the virus has been spreading in aquaculture and natural environments through international trade of CEV infected koi/carp (Way et al. 2017). To accelerate further research on this virus, we determined the full-genome sequence of CEV (Mekata, Kawato, and Ito 2021). The CEV genome size was estimated as 456K bp with 392 predicted genes. Ornamental fish diseases can impact fishes in natural environments. For example, infectious spleen and kidney necrosis virus (ISKNV), one of the genotypes in Megalocytivirus in the family iridoviridae, was isolated from ornamental fishes, such as angelfish and dwarf gourami (Kawato et al. 2020; Rimmer et al. 2017). An additional study revealed that indigenous fishes in Australia, silver sweep and Murray cod, were also susceptible to the virus (Go and Whittington 2019; Rimmer et al. 2015). The data indicated that transmission of the virus may have occurred possibly from ornamental fishes to wild fish. Also, in case of CyHV-2 outbreaks in Prussian carp in natural environments in Europe (Daněk et al. 2012) this might have been the cause. Therefore, clear communication to fish hobbyists (Figure 3), warning them, that their ornamental fishes may be infected with fish pathogen(s), and explaining, that any release of such infected fishes into natural environments may kill wild fish populations, is crucial. This means, communication between fish disease professionals and branch organisations, like the EAFP, ornamental fish branches, and ornamental fish hobbyists and their societies is recommended, apart from contact with wild fish boards, angling societies, who may help trace any mortalities in wild fish.
Q & A
O. Haenen asked if any other lab already used the above PCR method for CyHV-2 diagnostics on samples? Ito answered, this is not the case on these regions of the genome, because these data are still new. - D. Scarfe stressed that every importer of ornamental fish should ask for a health certificate as a preventive measure when receiving fish that could be diseased or infected, also on individual level when adequate. Followed a short conversation on any availability of international certifications, and on the OIE contribution in this regard.
Review of treatments against common ectoparasites in freshwater ornamental fish
C. M. Dover5, E. Schaefer9, A. Comas9, R. Preuss9, B. Gorgoglione1
Ectoparasites are a common causes of disease in freshwater ornamental fish, particularly in high-stress conditions such as high stocking densities, poor water quality, poor husbandry, and polymicrobial infections (Intorre et al. 2007; Hadfield and Clayton 2011). Fish from the global ornamental fish industry may or may not receive ectoparasite treatment prior to transportation, and quarantine is essential to prevent the spread of pathogens between fish stocks in the retail setting. We reviewed some of the most common ectoparasites which affect a wide range of freshwater ornamental fish species, as well as treatment options that could be applied in a retail setting. These ectoparasites included Ichthyophthirius multifiliis, Trichodinids, Tetrahymena sp., and gill (Dactylogyrus sp.) and skin (Gyrodactylus sp.) flukes. A brief review of bath treatments available against the selected ectoparasites showed that treatment options for freshwater ornamental fish remain limited, and dosages are frequently extrapolated from aquaculture research. Additionally, there is often a heavy reliance by hobbyists and retailers on commercial products of undefined compositions which may lack precise dosages and information on target species and efficacy. These products may also pose concerns regarding their toxicity, safety, and disposal. Research into the efficacy of phytotherapies has expanded due to their appeal from a safety standpoint (Valladão, Gallani, and Pilarski 2015). This externship project investigated bath treatments that could be readily applied by aquatic veterinarians in a retail setting by testing selected treatments in a commercial stock of fantail goldfish (Carassius auratus) during their quarantine upon arrival in a pet shop from their farming source in Thailand, and after 5 days of acclimation. Naturally-occurring infections with Gyrodactylus sp. flukes were confirmed with morphological identification via light microscopy prior to allocation of fish to each treatment tank. Selected treatments were based upon their ease of administration, efficacy, and practicality and consisted of 24 h baths of FMC (10 ppm) (Haenen et al. 2016), Potassium permanganate (3.6 ppm), Praziquantel (13.5 ppm, in 229 ppm Ethanol), and Levamisole (10 ppm), while using Ethanol (229 ppm) as a drug vehicle control. Baths were administered at day 0, 3, 7, and 10, with mucus scrape sampling occurring prior to each treatment and lastly on day 17. Samples were examined through light microscopy for ectoparasites, allowing the retrieval of Gyrodactylus sp. and Trichodina sp. (Figure 4). All treatments were easily applied and well tolerated by the goldfish, but none of them resulted in 100% efficacy. Over our 3-week treatment trial, a few distinct trends emerged. Firstly, Levamisole potentially showed the most promising results in reducing Gyrodactylus sp. flukes, as these numbers declined steadily over the course of this study. Secondly, the number of trichodinids detected increased over the course of the experiment, except in the tanks treated with potassium permanganate, in which no trichodinids were noted. Generally, a small number of fish in each tank had the highest numbers of ectoparasites, which may indicate that it might be more effective for hobbyists or retailers to single out fish with high ectoparasite burdens for individual treatments. Further work is required to evaluate the statistical significance of each treatment, as well as the effects of co-infections with Gyrodactylus sp. flukes and trichodinids.
Q & A
O. Haenen asked if it was also tested using Pyceze® (bronopol), a drug against fungi and protozoan ectoparasites, which was presented during the Dublin EAFP Conference in 2001. C. Dover replied that they did not find enough literature in this regard at the time of drug selection for the study. Furthermore, she has not seen any bronopol product on the pet store shelves. C. Dover stated that it would be definitively interesting to use bronopol in further tests. D. Verner-Jeffreys provided a source link to the Pyceze® product characteristics (www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_131969.PDF)
Antimicrobial susceptibility of bacteria isolates from intestinal flora of dwarf gourami (Colisa lalia)
S. Menanteau-Ledouble10, P. Grippon6, V. Demuth10, L. Le Devendec6, E. Larvor6, A. Werlberger10, E. Jouy6, I. Kempf10, M. El-Matbouli10, S. Baron6
The exchange of live fish occurs mainly between Asian countries towards the USA and Europe - the two main importers - (Sicuro et al. 2020). Misuse and overuses of antibiotics in this non-food producing aquaculture select resistant bacteria while international trade facilitates their dissemination. Several bacterial isolates from ornamental fish have zoonotic potential, and there are many reports regarding the isolation of zoonotic pathogens from aquarium fish and water, as well as human bacterial infections associated with aquarium keeping. In our study, 42 bacterial isolates were recovered from the intestinal flora of seven dwarf-gourami. We identified these isolates and characterised their phenotypic susceptibility to investigate their potential role in antimicrobial resistance (AMR) dissemination. A panel of 29 agents, belonging to 11 classes of antibiotics listed as critical agents for Human health (WHO list) or potentially used in aquaculture (CLSI, 2020), were tested using the agar diffusion method (CLSI, 2015, 2016). A primary aim of susceptibility testing is to categorise isolates into two groups for each antibiotic tested, based on their breakpoint values (BV): isolates are categorised as wild-type (WT) or non-wild-type when epidemiological cut-off values (ECV) are used, or resistant vs susceptible when clinical breakpoint value (CBV) are used. Among the 12 genera identified, the four most abundant were Citrobacter (38.1%, n=16), Aeromonas (19.0%, n=8), Bacillus (16.6%, n=7), and Pseudomonas (9.5%, n=4), while the other genus were only represented by one isolate. Inhibition zones were measured 1218 times (29 antibiotic tested * 42 isolates). However, categorisation was only possible for 525 measurements due to the lack of BV, and over half of the data (56.8% n=693) could not be interpreted. Thus, concerning Citrobacter, BV were available for 20 out of the 29 antibiotics tested, covering eight classes of antibiotics. Highest frequencies of resistance were observed for quinolone and betalactamin classes, 93.7% (n=15) and 75% (n=12). Multidrug resistant isolates (resistant to at least three antibiotic classes) were detected in four isolates, all belonging to the genus Citrobacter. One of these isolates was resistant to six classes of antibiotics. Nevertheless, the number of MDR isolates was likely underestimated due to the limited number of antibiotic classes for which BV are available. This study illustrates the complexity of AMR testing for the microflora of ornamental fish due (1) to the diversity of the bacteria species, and (2) methodologic gaps. CBV aim at predicting the likelihood of a successful treatment. These values depend on the bacteria, the antibiotic, and the host. In our case, the majority of CBV used are those defined for humans, and these could be used in case of human infections, but they are not suitable for infections in fish. Moreover, to investigate the dissemination of AMR, the accurate value is the ECV, which separates bacterial populations between wild type and non-wild type bacteria. The non-wild type bacteria are those that have acquired a resistance mechanism to one antibiotic. For the moment, ECV are still not widely available, and improvement and standardisation of phenotypic methods are needed to investigate AMR in ornamental fish and more generally within the fish microflora.
Q & A
O. Haenen asked if we should avoid using fluoroquinolones for ornamental fish, because of the very high resistance shown of Citrobacter freundii? S. Baron replied, this depends: in case the resistance genes would be non-motile, there would be low risk. Research should further prove. - D. Verner Jeffreys remarked that the presented fish isolates of Aeromonas were much less resistant than those of CEFAS, asking why. S. Baron replied that this could have been caused by the source of the fish or the bacterium, although that is hard to say. S. Menanteau-Ledouble remarked, that the bacteria were isolated from the intestine of the dwarf gourami and cultured on Trypton Soy Agar and Mueller Hinton agar.
Intra vitam detection of Cyprinid herpesvirus 3 (CyHV-3) in koi carp for export from Japan
K. Yuasa4 and T. Ito4
In Japan, each farm, registered as listed establishment by the Ministry of Agriculture, Forestry and Fisheries (MAFF) can export koi carp. The registered farm is required to conduct testing for CyHV-3 (aka Koi herpesvirus, KHV) and Spring viraemia of Carp (SVC) virus twice a year for more than 2 years, which is based on MAFF provided guidelines for exporting koi carp. When the farm attempts to introduce koi carp from other listed establishment, the farm shall get a health certificate of the establishment issued by the prefecture. In accordance with the Act on Securing Sustainable Aquaculture Production, when the owner of a farm notes abnormalities such as a mass mortality, the farm shall immediately report this to the representative department of the prefecture. Koi carp in the establishment where KHV has been confirmed shall be restricted to be moved to another establishment or get sacrificed. However, the number of annual KHV cases in Japan gradually increased from 2015 to 2018. The number in 2018 was 42, which included 6 cases at koi farms, based on MAFF archives. The MAFF conducted field surveys at koi farms where KHV occurred in 2018 to determine the cause of viral contamination in the farms, which was followed by a revision of the guideline for exporting koi carp by MAFF in 2019. Main items of the revision are strengthening of biosecurity in establishments of the farm and defined sampling areas that shall include all ponds/aquaria for rearing fish for exportation. Some koi exporting farms preferred the cohabitation assay, in which KHV detection is attempted from fish cohabited with target fish for the test, for the twice a year tests. Therefore, we evaluated the viral detection efficiency of the cohabitation assay in comparison with PCR detection assay with the gills or scales sampled by biopsy using fish periodically sampled after viral exposure with KHV. In addition, PCR with the gill tissues and mucosal tissues on the body surface taken by swab were examined to compare the viral detection efficiency. Results indicated that a PCR on gills and scales can detect KHV for apparently longer periods than the cohabitation assay does. PCR on the mucosal tissues is also inferior to the PCR on gills or scales at the subclinical period. The MAFF has recommended to use the PCR assay on gills sampled by biopsy for the test of koi carp for exportation rather than by cohabitation assays, referring to the results. After the introduction of reconsidered manners to control KHV, the number of cases of KHV in Japan has been decreasing up to now (Figure 5).
Q & A
N. Gagné remarked, that KHV is still detected by qPCR, but it could not be detected in water (eDNA) - do you have a good correlation between eDNA values and qPCR values in tissues? And if so, the cohabitation shows that the virus is still transmissible but not detectable anymore in water samples, so, is this indicating a lower sensibility of the eDNA method? It is interesting work, after a while, fish seem to shed a low amount (at 77 days KHV detection in gill and body mucus is low). K. Yuasa answered that the tissue DNA-method is much more reliable than the eDNA method from the water; they recommend to sample gills.
Persistent like carp edema virus – a case study of koi sleepy disease affected fish population
M. Adamek7, F. Teitge7, A. C. Miebach7, D. Steinhagen7
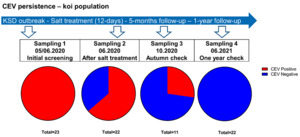
Carp edema virus (CEV) is a very successful fish poxvirus with worldwide distribution causing a serious disease called koi sleepy disease (KSD) in koi ornamental variety of common carp (Way et al. 2017). The spread seems to be driven by international trade of koi and could be aided by the ability of the virus to persist in the host after acute infection or natural immunisation performed by some fish producers (Miyazaki, Isshiki, and Katsuyuki 2005). Poxviruses of higher animals like poultry are known for their persistence in the environment and the ability to re-infect their hosts at a suitable time point. In fish, this problem was relatively under-studied. Here we present available, already presented results and new data from Germany, which could shed some light on answering the question of whether CEV can induce persistence. For example, testing of several shipments of koi, which were sent to German retailers from Japan, showed that asymptomatic fish were repeatedly found positive for the virus (Adamek et al. 2016). Furthermore, observation of fish and sequencing of the virus from some fish farmers in Germany showed that over the period of 2014-2021 repeating outbreaks of KSD were caused by the same virus variants, which could hint that the virus persisted in the population (Adamek et al. 2021). However, the experimental infections did not confirm a transfer of the virus from survivors of the CEV infections (Adamek et al. 2017). This may indicate that fish, at some point, are capable of mounting successful immune responses. When this happens, the virus is cleared from the fish and loses its ability to persist in the population. Finally, the newest, unpublished data from an ornamental fish population consisting of 23 koi, which was followed-up after an outbreak of koi sleepy disease (KSD) for a year, confirm long persistence (Figure 6). All 23 fish were confirmed to be positive for CEV by qPCR during the outbreak. Immediately after diagnosis, a 12-day long salt bath (0.5%) was administered, which led the clinical signs to subside, and only a single mortality occurred. However, after this treatment, 14 from 22 fish remained positive for CEV, while being free from clinical signs. During a routine autumn health check, about five months after the outbreak no clinical signs were noticed, although three from 11 fish tested remained CEV positive. The virus could not be detected after one year from the outbreak (Figure 6), which could suggest that eventually the fish were able to clear the infection. Combining the above observations, it could be concluded that CEV seems to be persisting in fish for at least several months. There is a subset of fish, which are not able to clear the virus for months, become long haulers of the infection without presenting any clinical signs. This has special significance for the ornamental fish trade where some producers offer naturally immunised fish, which were exposed to the virus and then treated by salt bath and/or increased temperature of water. This procedure could produce persistent carriers of the virus, which could explain the successful spreading of this pathogen. These findings should underline the imperative for checking fish before purchase, as the virus seems to be difficult to be cleared from a susceptible population.
Q & A
T. Ito asked if the fish was resistant against CEV after 1 year? M. Adamek replied that they did not test, as these were too expensive fish, nor did they take blood samples. - B. Gorgoglione asked about the assessed efficacy of salt treatment against CEV. M. Adamek replied that this treatment still seems effective: 0.5% salt causes a halt to mortality of diseased carp/koi, but depending on the insurgence of secondary infections, which makes the salt treatment work less effectively. - E. Trani asked about the mechanism of action of salt treatment? M. Adamek replied that this treatment balances the loss of ions, which is a characteristic clinical sign for koi sleepy disease.
Workshop Discussion
The final general discussion started with online polls asking for the opinion of the workshop attendees on which organisation should move forward to promote regulatory actions in the sectors of ornamental and laboratory fishes.
O. Haenen pointed out as the collaboration between certain organisations was mostly chosen. - D. Scarfe mentioned the value of the contribution provided by K. Yuasa, developing non-lethal sampling for CyHV-3 in Koi. Furthermore, he admired, Japan is trying to eradicate CyHV-3. K. Yuasa clarified that data presented are in process of publication. - D. Scarfe asked Stinton about certification (health vs inspection certifications available/required) regarding specific diseases of ornamental fishes. N. Stinton replied that it is hard to estimate their reliability regarding guarantees these certificates may give or not give based on regional status. It also depends on the set up of the receiving country, visual inspection is mostly the only thing, apart from certificate check. – O. Haenen asked for the format of a health certificate used for the UK. N. Stinton replied that this differs in each country. Thus, O. Haenen underlined that the OIE has formats, at least for edible fish, and these generally work fine. D. Scarfe supported and recommended that the OIE should design a certificate for ornamental fish exportations. – B. Gorgoglione asked if young vets receive enough education about aquatic species so they could take the health/disease diagnosis task on ornamental and laboratory fishes. N. Stinton replied, that this field is still mostly under-focused, and should be better highlighted in the veterinary curricula.
O. Haenen remarked, once again, that collaboration between certain organisations is needed. She highlighted, the Fondazione Guido Bernardini organised a course ‘Pathology of laboratory fish’ in 2018 at Leiden University Medical Centre, the Netherlands. Further organisations were recommended as well: E. Riera-Ferrer mentioned that associations like the AALAS (American Association for Laboratory Animal Science) (www.aalas.org/), FELASA (Federation of European Laboratory Animal Science) indicating their work on “Health monitoring for fish in research” (www.felasa.eu/working-groups/id/5), could act together with experts from the EAFP. Whipps also mentioned the Zebrafish Husbandry Association (www.zhaonline.org/).
O. Haenen concluded that both in laboratory and in ornamental fishes the issues raised during this workshop would need urgent attention, hoping that this request will be somehow picked up by suitable organisations. She thanked all the co-organisers of this EAFP workshop, all speakers and participants, and the conference support for this virtual workshop, thereafter, closing the workshop session.



_*gyrodactylus*_s.png)




_*gyrodactylus*_s.png)

