Introduction
Non-infectious pathologies in fish are of great interest to pathologists, and some diseases are very similar and present an evolution and biological behavior similar to mammals and humans, so that today we have an important amount of experimental models of human diseases in fish (Masahito, Ishikawa, and Sugano 1988). Among these diseases, neoplasms are particularly intriguing because of their similarity to those found in vertebrates, thus marking an interesting phylogenetic relationship. There are bioinformatic models that analyze the phylogeny of neoplasms in vertebrates (Schwartz and Schäffer 2017). A large number of neoplasms diagnosed in humans have a counterpart in fish (Mawdesley-Thomas 1971). Some are generated by similar carcinogens, such as HCC, induced by chlorinated organs, algae toxins (microcystin), and mycotoxins (aflatoxin) (Malins and Ostrander 1994; Pereira 2001; Labine et al. 2015; Saha Turna and Wu 2019). In humans, primary liver cancer is the second leading cause of cancer-related death worldwide and therefore a major public health challenge (Global Burden of Disease Cancer Collaboration 2018).
The specimens of O. bonaerensis studied here came from a highly eutrophic lake, where, along decades, frequent blue-green algae blooms (Anabaena and Microcystis) were registered and their relative amount is relatively high (Herrero, Sosnovsky, and Morbillo 1998; Morbillo, Sosnovsky, and Herrero 1998). More recently, intense proliferation of the dinoflagellate Ceratium have occurred (Marozzi 2019). In the 90’s, various histopathological lesions have been reported in various species of fish, including O. bonaerensis that inhabit Lake San Roque (Romano et al. 1998).
Cyanobacteria blooming events that produce natural toxins occur in fresh waters around the world, although the potential risk of many cyanobacteria metabolites remains unknown. Only microcystins, a class of cyanopeptides, have been studied intensively, and the large amount of evidence on exposure concentrations and toxicity has led to their inclusion in the water quality risk management model (Janssen 2019).
Materials and methods
We have examined 100 specimens of pejerrey Odontesthes bonariensis from Lake San Roque (31°23’S, 64°28’W), Cordoba, Argentina, captured in seven days. The average length of the animals was 21.7 +/- 1.11 cm and the average weight was 217.3 +/- 1.2 g. All specimens were euthanised in an immersion bath with MS-222 of 100 ppm (Western Chemical, USA). The necropsies were performed following a previously described protocol (Romano, Sardella, and Russomando 1987). Fragments of the neoplastic and macroscopically normal liver lesions were fixed in 10% buffered formaldehyde, included in paraplast, sectioned at 3 μm, and stained with hematoxylin and eosin and with Masson’s trichromic.
For the immunohistochemistry, the histological sections were stained with an Avidin Biotin Peroxidase complex modified technique (Hsu, Raine, and Fanger 1981). After dewaxing and hydration, the sections were washed in water for 5 min, exposed to a 1% hydrogen peroxide solution in methanol for 30 min to block endogenous peroxidase activity, and washed in phosphate buffered saline (PBS), pH 7.2, for 20 min. After blocking, they were incubated with 3% bovine serum albumin in PBS (BSA, type V Sigma Chemical, St. Louis, Mo.USA) for 40 min. The sections were incubated for 60 min with a primary anti-hepatocyte specific antigen antibody (Hep Par 1) (Roche, Argentina), for 45 min with CD34 antibody (Roche, Argentina), for 40 min with CD133 antibody (Roche, Argentina), and for 90 min with S100 antibody, at a dilution of 1:2500, 1:2000, 1:1500, and 1:1100 in PBS, respectively. Subsequently they were washed in PBS and exposed for 45 min to the biotinyl avidine peroxidase complex (Vectastain ABC kit, Vector). The sections were exposed for 7 min to a 0.1% solution of diaminobenzidine (Polyscience, Warrington, Pa., USA) which was added before use, 0.2% oxygenated water in 50 mM tris buffer, pH 8. Fragments of a human HCC, belonging to the Pathology Department of the Piñero Hospital in Buenos Aires, Argentina, were used as a positive control. As a negative control in all cases the primary antibodies were replaced by normal rabbit serum at a similar dilution according to Mills (1992).
Tissue fragments of all HCC were fixed for transmission electron microscopy (TEM) in 0.2% glutaraldehyde and post-fixed in osmium tetroxide, then prepared according to the protocol described in previous work (Luchini, Wicki, and Romano 2015). Thick sections stained with toluidine blue were used to locate tumor areas. Ultrafine sections were then prepared and stained with uranyl acetate and lead citrate and examined under a Jeol JEM-8T electron microscope (Jeol, Tokyo, Japan).
Results
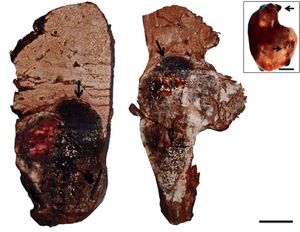
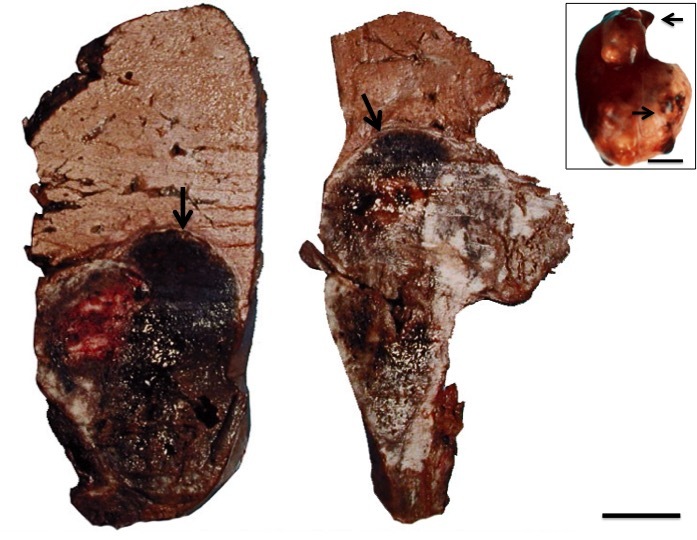
In the necropsies, hepatomegaly was found in 24 specimens with external irregular nodular lesions. On cutting, nodular lesions with necrotic and hemorrhagic areas were observed (Figure 1).
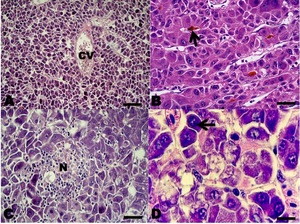
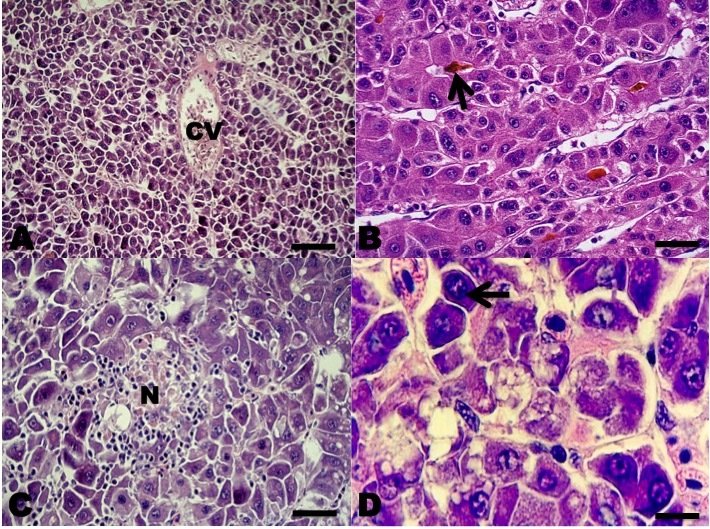
In the sections belonging to the human HCC (positive control), all the antibodies used were marked in the same way as in the fish HCCs. In the sections where the primary antibodies were replaced by normal rabbit serum (negative control), no staining was observed. The HCC neoplasms were classified according to the WHO’s criteria for human HCC (Bosman et al. 2010; El Jabbour, Lagana, and Lee 2019). According to WHO criteria 20 neoplasms presented a histopathological pattern typical of the conventional HCC type, in which a proliferation of liver cells similar to the adult hepatocytes was observed, with little anaplasia, few mitoses, but with changes in the normal liver tissue architecture.
Among the neoplasms, four presented a histopathological pattern of sarcomatoid HCC, in which a proliferation of spindle-shaped cells was observed; few with anaplasia and isolated mitoses with little resemblance to normal hepatocytes, and nests of spindle-shaped cells with fibrosis separating neoplastic lobes (Figures 2 and 3).
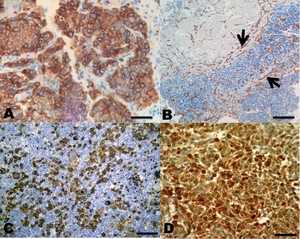
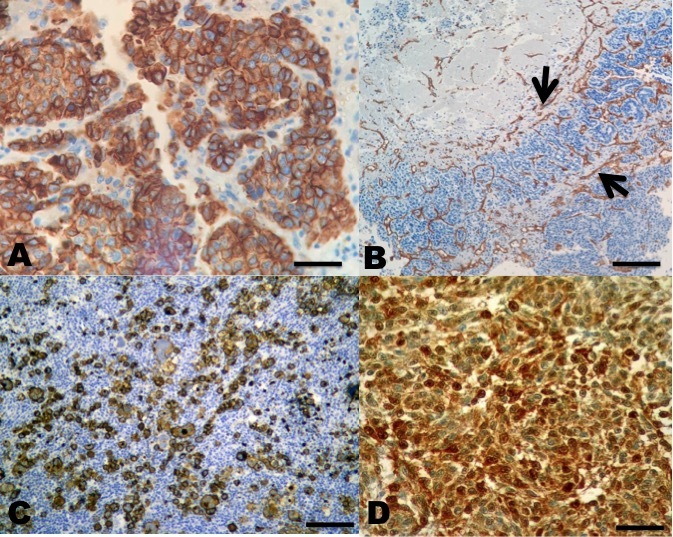
In the immunohistochemistry, both Hep Par 1, CD34, CD133, and S100 antibodies were positive in all types of HCC (Figure 4).
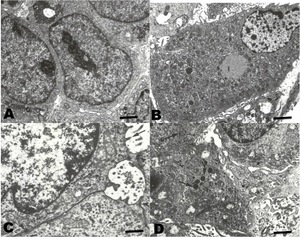
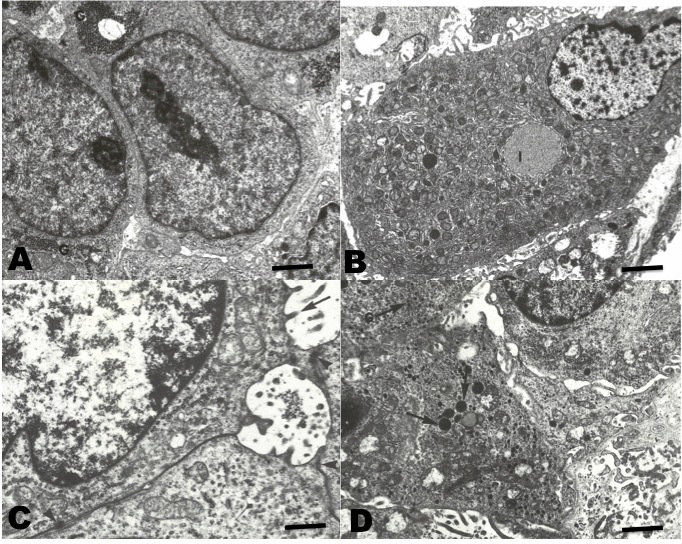
In TEM (Figure 5), the neoplastic cells of conventional HCC resembling the normal liver cells. Abundant endoplasmic reticulum and mitochondria were observed. These cells showed aggregates of glycogen in the cytoplasm. Occasionally, clear cells with abundant lipid vacuoles were observed. In the sarcomatoid HCC, spindle cells with numerous osmophilic granules were observed. In addition, mucin-like vacuoles were observed. Isolated narrow cell junctions and microvillus-like prolongations were observed.
Discussion
The fourth edition of the World Health Organization (WHO) classification of tumors of the digestive system was published in 2010. In this edition, besides the conventional hepatocarcinoma (HCC), the WHO recognised the following 5 morphological subtypes of HCC: fibrolamellar HCC (FL-HCC), scirrhous HCC (S -HCC), undifferentiated carcinoma, lymphoepithelioma type carcinoma, and sarcomatoid HCC (Bosman et al. 2010; El Jabbour, Lagana, and Lee 2019). The majority of the studied cases (77 %) corresponded to conventional HCC, and 23 % to sarcomatoid HCC. In fish, neoplasms in general are not very frequent, but lately several types of neoplasms have been described in different fish species (Vergneau-Grosset, Nadeau, and Groff 2017). Several cases of HCC have already been described, but due to the low frequency of these neoplasms they have not been classified into histological types, and in the revised bibliographies the HCC subtype has hardly been mentioned. According to Roberts (2012), digestive system neoplasms in general and liver neoplasms are not very frequent. Most articles on HCC are related to experimental models associated with chemicals (Falkner, Emdin, and Ostberg 1976; Aoki and Matsudaira 1977; Wang et al. 2010; Wrighton, Oderberg, and Goessling 2019). Majeed, Jolly, and Gopinath (1984), reported 38 cases of HCC in rainbow trout (Oncorhynchus mykiss) from farms, which the authors associated with aflatoxin-contaminated food.
Both well-differentiated hepatocellular neoplastic lesions and poorly differentiated ones represent a diagnostic challenge. “Well-differentiated” implies that the histomorphology of the tumor is very similar to the native tissue from which the tumor originates. Thus, a well-differentiated HCC recapitulates normal liver tissue, mimicking the following benign and preneoplastic entities: hepatocellular adenoma, focal nodular hyperplasia, and regenerative nodule (El Jabbour, Lagana, and Lee 2019). In order to detect malignancy in an apparently normal and well-differentiated hepatic tissue, immunohistochemistry plays a key role in the differential diagnosis, such as application of the monoclonal CD34 antibody, that shows diffuse sinusoidal capillarisation typical of malignant neoplasms (Cui, Wu, and Wen 2018). Both conventional HCC and sarcomatoid HCC showed a histopathological pattern similar to mammals and humans (van Sprundel et al. 2014; Pittman and Brunt 2015; Rastogi 2018).
A panel of positive monoclonal antibodies, also known as anti-Hep-Par1, anti-CD133, anti-CD34, and anti-protein S100 (this was expressed in sarcomatoid type HCC) contributed to the differential diagnosis between benign and malignant lesions, as observed with the anti-hepatocyte specific antigen (Wennerberg, Nalesnik, and Coleman 1993; Torbenson, Y, and Yeh 2018). Lately, immunohistochemistry has represented an important diagnostic tool in the identification of the histiogenesis of neoplasias in fish (Romano et al. 2018, 2020; Iaria et al. 2019). Still, immunohistochemistry in these cases is neither 100% specific nor 100% sensitive. Thus, a careful review of histomorphology and the rigorous application of the cytomorphological criteria of malignancy, in addition to the phenotypic expression of the marked antigens, is essential to establish a consistent diagnosis of HCC (Choi, Ramachandran, and Kakar 2017).
CD34 is a glycosylated transmembrane protein that belongs to the salivary mucin family associated with neovascularisation, and is currently considered to be closely related to the development of tumor blood vessels. Therefore, CD34 can be detected in some types of tumors that are associated with blood vessel proliferation (de Boer et al. 2000).
The anti-hepatocyte specific antigen, also known as anti-Hep-Par1, recognises benign and malignant liver derived tissues, including tumors such as HCC. It recognises both normal adult and fetal liver tissue. The typical pattern is a granular cytoplasmic stain. This antibody is useful for differentiating hepatocellular carcinomas with adenoid characteristics from adenocarcinomas, whether primary in the liver or metastatic lesions in the liver (Minervini et al. 1997; Zimmerman et al. 2001; Kuroda and Yorita 2018).
The expression of CD133 is regulated by many extracellular or intracellular factors and represents cellular type changes with particular functions (Wu and Wu 2009). The transforming growth factor β1 (TGFβ1) is able to regulate the increase of CD133 expression specifically within the cellular line of HCC (You, Ding, and Rountree 2010).
During the last decade, it has been discovered that through comparative and functional genomics that several S100 genes are expressed differently in cancer cells. In general, poor tumor differentiation and aggressive neoplasms (Maletzki et al. 2012) have followed the overexpression of S100.
The ultra-structural characteristics of conventional HCC have been widely described for both human and other mammalian HCC (Lapis 1988; Grab, Skubleny, and Kneteman 2019). The cells contain abundant rough endoplasmic reticulum, a relevant number of mitochondria, and glycogen and lipid vacuoles. These utrastructural characteristics are similar to those found in normal hepatocytes (Baratta et al. 2009). On the other hand, sarcomatoid HCC, also called fibrolamellar, has presented cells which are very different from those of normal hepatocytes; being spindle-shaped, with electrodense granules, and with narrow cell junctions (Andreola, Audisio, and Lombardi 1986; Hasegawa 1996). In all the cases reviewed in this work, they showed an ultrastructure similar to those described in the literature for HCC in mammals and humans.
Regarding the etiology of HCC, in mammals and humans, most cases are related to previous hepatic pathologies, such as cirrhosis and hepatitis B and C, with post-necrotic cirrhosis (Wilson, Mann, and Borthwick 2017; Li et al. 2016; Lin and Kao 2018). Also in mammals, chemical carcinogens are related to the development of HCC (Yang et al. 2019). Non-alcoholic liver metabolic diseases and exposure to diet toxins such as aflatoxins and aristolochic acid are risk factors that can generate HCC (Turner et al. 2005). In fish, in contrast, HCC most often is caused by chemical carcinogens of organic or inorganic origin. Aflatoxins are mycotoxins, which contaminate many basic cereals and oil seeds, being well recognised as liver carcinogens in fish (Anikuttan et al. 2018). Aflatoxin contamination is widespread in areas with a high incidence of HCC. The main form of aflatoxin involved in hepatic carcinogenesis is aflatoxin B1 (AFB1), produced by Aspergillus sp. (Wild, Miller, and Groopman 2015).
Cyanobacteria are also considered potential hepatocarcinogens (Svircev et al. 2009) and cyanotoxins represent a very diverse group of toxic substances. They are either attached to the membrane or exist free within the cells. The release of toxins occurs during cell life, but mainly after cell death through passive release of the cell content. Cyanobacterial toxins include neurotoxic alkaloids (anatoxin-a, anatoxin a(s), saxitoxins), hepatotoxic peptides (microcystins), and the hepatotoxic alkaloid (cylindrospermopsin) (Fitzgerald 2001).
Hepatotoxic cyclic peptides include nodularine and different forms of microcystins. Microcystins are a large group of cyclic peptides with varying amino acids in 7 different positions, representing approximately 60 different forms. The most toxic form is microcystin-LR. The toxicity of microcystins is due to their strong union and inhibition of phosphatase proteins (Hitzfeld, Hoger, and Dietrich 2000; Chorus 2001). It has been demonstrated that after intraperitoneal microcystin injection, the toxin is rapidly found in the liver (Nishiwaki et al. 1994). The presence of microcystin has also been demonstrated in the ovary and liver of fish (Qiao et al. 2019). In mammals the toxic action of microcystin has been described and possibly, its mode of action does not differ from that in fish (McLellan and Manderville 2017). In this work we described 26 cases of HCC in O. bonariensis from 100 specimens caught in a highly eutrophic lake, with a history of continuous cyanobacteria blooms. The HCC observed were classified according to WHO, with 20 being conventional HCC and 4 sarcomatoid HCC. The immunohistochemistry confirmed their malignant character and histiogenesis.
In this work, the detailed recognition of the tissue and cellular structure of the neoplasms proved an important tool for diagnosis in addition to immunohistochemistry. Although electron microscopy provided a less-specific methodology for the objectives of the study, the ultrastructural findings were as expected and were useful to support the histopathological diagnosis.
The etiology of these HCC could not be confirmed, but considering the chronic presence of cyanobacteria with frequent algal blooms, we suggest that they are responsible for these neoplasms, especially considering the hepatic carcinogenic activity of algae peptides such as microcystin.
Acknowledgements
This study was supported by the research funds from MCT/CNPq - Project #301245/2016-09 MCT/CNPq/CT- Agronegocio/MPA Public Notice 036/2009 Project #308013/2009-3, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Ministério da Pesca e Aquicultura (MPA). L.A. Romano is a research fellow of the National Council for Scientific and Technological Development, 301245/2016-9.