Introduction
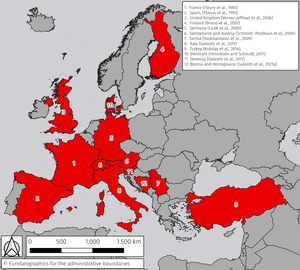
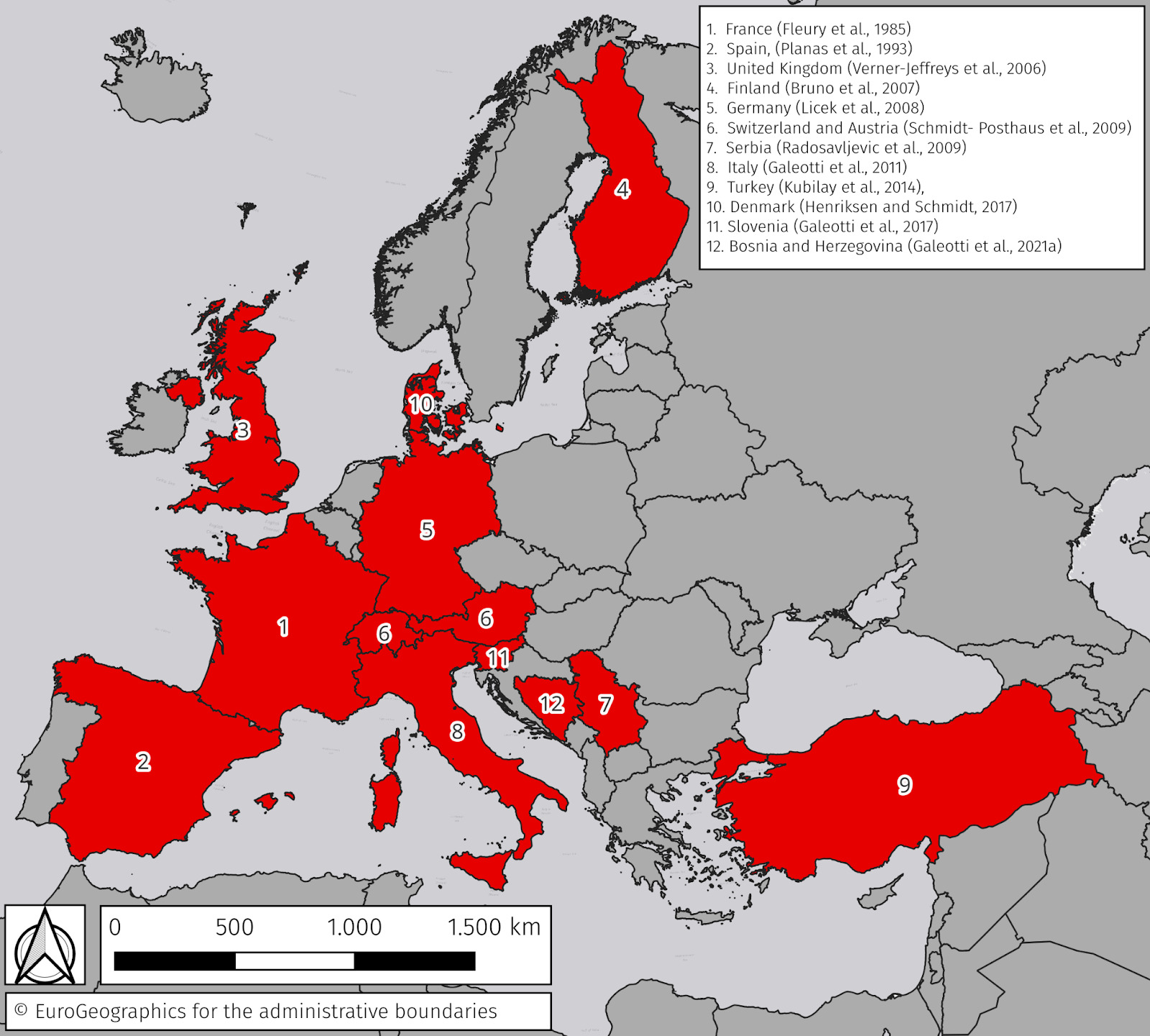
Red mark syndrome (RMS) is a widespread, self-resolving skin disease associated with the presence of a Midichloria-like organism (MLO) (Metselaar et al. 2022). The first appearance of a syndrome with clinical signs and histopathological findings consistent with RMS dated back to the late 1960s in the USA (Erickson 1969), and the first described outbreak possibly linked to an individual batch of eggs (Nevada Department of Wildlife 1974). The condition was described in Europe in the 1980s (Fleury, Vuillaume, and Sochon 1985; Planas et al. 1993) and then subsequently reported in the United Kingdom (UK) in late 2003 (Verner-Jeffreys et al. 2008). Since then, the disease has rapidly spread to the rest of Europe (Figure 1).
To date, this syndrome has only been reported in rainbow trout (Oncorhynchus mykiss) both in juvenile stages and in larger sizes, in flow-through and recirculating aquaculture systems (RAS) (Verner-Jeffreys et al. 2008; Jørgensen et al. 2019; Metselaar et al. 2020; Orioles, Saccà, et al. 2022). Cohabitation challenge models established under experimental conditions have also allowed the condition to be reproduced in an experimental setting (Erickson 1969; Oman 1990; Verner-Jeffreys et al. 2008; Jørgensen et al. 2019). Presumptive diagnosis is mainly based on clinical features, while confirmatory diagnosis is based on skin histology and molecular biology techniques such as quantitative and digital droplet PCR (Oidtmann et al. 2013; Galeotti et al. 2016; Galeotti, Sarli, et al. 2021; Orioles, Bulfoni, et al. 2022). Unfortunately, a confirmatory diagnosis is rarely performed.
Existing epidemiological data of RMS consists of several reports of outbreaks without any structured study estimating its prevalence at a national or European level. The evidence of the current economic impact of RMS on trout aquaculture remains unknown, but it is well-recognized as challenging for the industry (Metselaar et al. 2022). In particular, producers farming portion-size trout (300-500g) and organic seem to be more affected by RMS. In these production settings, the impact of RMS has been considered high, with more than 5% of their production costs attributed to this condition (Verner-Jeffreys and Taylor 2015).
Rainbow trout is Italy’s most widely farmed freshwater species, with a total annual production of close to 35,000 tons (FEAP 2020). Three hundred and sixty farms, mainly concentrated in the north of Italy, account for about 70% of total production. Within the Italian freshwater fish farming context, organic production is a growing sector (ISMEA 2021; D’Agaro, Gibertoni, and Esposito 2022).
This epidemiological study aims to estimate the prevalence of RMS in Italian rainbow trout farms through a questionnaire-based survey and is an attempt to define more precisely the distribution and the impact of this disease on a national level in Italy.
2. Methods
Data were obtained from a short questionnaire designed to estimate the presence of RMS between 2020-2021 in Italian rainbow trout farms (Figure 1). The questionnaire was based on a version (Schmidt, Thompson, and Padros 2018) previously used in the Danish rainbow trout sector.
Three hundred and twenty site managers and field veterinarians (one for each site) were contacted through API (Italian fish farmers association), SIPI (Italian association of fish pathologists), or directly (in person or by phone) during late 2021 and early 2022. Dr Massimo Orioles, University of Udine (Lead author) interviewed the farmers and veterinarians and collected the data.
The questionnaire was composed of 17 questions including a combination of open (2), closed (9) and multiple-choice (6) questions. The questions were based on general characteristics of the farm (name, type of farm and production, geographical localization, water source), and on specific details about RMS outbreaks, including costs associated with the disease and therapeutic intervention. Details included water temperature, season when the outbreak was observed, size of fish affected, number or percentage of tanks effected, number or percentage of fish effected within each unit, type of treatment used, possible source of infection and potential recurrence of the disease in the same batch of fish. The case definition for the presence of RMS includes the appearance of focal to multifocal chronic hyperemic, usually raised, non-ulcerative lesions between 5 mm to several cm in diameter; affected fish were generally in good condition, with no mortality and show normal behaviour (Oidtmann et al. 2013). Furthermore, the farmers and veterinarians were asked whether diagnosis was made through laboratory analysis.
3. Results
A total of 109 (out of 320 contacted) rainbow trout farms responded to the questionnaire through their farm site health managers and consultant veterinarians. In 8 cases, the farm site health managers were personally guided through the questionnaire; in 11 cases farm site health managers responded by email; and in 90 cases the answers were provided by email from experienced field veterinarians regularly consulting the farm in the period in question. Out of the 109 responses, 83 were located across the north of Italy. The estimated total production of these 109 farms represented about 64% of the entire Italian trout production (approximately 34,400 tons - FAO 2019).
RMS was observed at least once in 31 out of the 109 sites during 2020-2021. Farms with reported RMS were distributed across 11 different regions (Figure 2).
Figure 2 illustrates the distribution of respondents among regions and the number of farms where RMS has been observed.
The 31 sites were all medium and large-sized farms, with a mixed production strategy based on both portion size trout products (300-500 g) and larger fish (more than 500 g). Water was predominantly sourced from rivers (27 out of 31 cases). During outbreaks, the water temperature was reported to fluctuate between 5° and 15°C.
Diagnosis in all cases was only based on clinical features and necroscopic findings. No further analysis was performed to confirm the diagnosis. In 12 of 31 (38.7%) cases, the disease was observed more frequently in the last five years. Most commonly [14/31 (45.1%)], 10-30% of tanks were affected, while in 10 cases (32.2%), the disease spread through more than 50% of the tanks.
In most cases (20/31 (64.5%), relatively few fish in a unit (up to 10%) showed typical clinical signs of RMS, while the presence of clinical signs was reported in 10-50% of the population in a unit in 11 (35.5%) cases. Most commonly, affected fish were close to commercial portion size (300-500g; 41.9% of cases) or somewhat smaller (100-300g; 29.0% of cases). In 7 cases (22.6%), RMS was observed in trout of less than 100g (all reported by the same veterinarian in the same region). RMS in trout >500g was only reported from two farms (9.67%).
Twenty respondents (64.5%) reported that recurrence in the same batch of fish was observed; in 22 cases (70.9%), the infection was believed to be transmitted through the water from upstream farms and in 8 cases (25.8%) from the introduction of infected fish or eggs. RMS lesions were most commonly observed in spring, and active RMS lesions were never observed during summer.
The farmers opted not to treat the disease in 14/31 (45.1%) cases, all of which were in cases where 0-10% of tanks were affected. Oxytetracycline was used in 14 cases (45.1%), with the majority used in most severe outbreaks, where more than 50% of units were affected, with 10-50% of the population involved. Treatment was given in 3 cases (9.6%), but details were not specified.
RMS-associated costs were unknown or determined in 10 cases (32.2%). In 10 cases, the respondents considered RMS-associated costs mainly related to treatments. Eight respondents out of 31 (25.8%) stated that this disease has no associated costs, while only in 2 cases, a commercial downgrade of carcasses was claimed as the only cost. In both cases, RMS was observed in over 50% of units affecting 10-50% of individual fish of all sizes.
No concerns over feed conversion, mortality or growth rate were raised. The percentage of RMS-associated production costs was never specified. Details of the results are summarized in Table 1.
4. Discussion
As reported by the results of the 25th Annual Workshop of the European Reference Laboratories for Fish Diseases (DTU-AQUA, Denmark, https://www.eurl-fish-crustacean.eu/fish/annual-workshop/26th-aw-2022/25th-aw-presentations), RMS remains to date a major concern in rainbow trout farming, even though the disease is generally considered manageable by health managers and veterinarians. This is the first survey of RMS conducted in Italy with the aim of estimating prevalence and distribution on the Italian territory. The survey was considered acceptable to represent the Italian sector as the responding farms, mainly concentrated in the north of Italy, were estimated to be around 64% (about 22,000 tons) of the total national rainbow trout production. We considered the content validity of the answers good, as the survey was already pilot tested in Denmark (Schmidt, Thompson, and Padros 2018), and the questions were concise and easy to understand, realistically covering all the information needed and obtainable from farmers and field veterinarians. Although no diagnostic confirmation through histology, nor molecular biology techniques was performed, thereby introducing a potential bias in the evaluation of the prevalence, RMS presumptive diagnosis was made mainly by experienced field veterinarians (the actual respondents in 82% of the cases). Despite this, the accuracy of these data remains essentially unknown, and most of the answers could be prone to recall bias, as they are not based on a specific recording system. Data described here should be interpreted with this in mind, and the use of data from computer-based management systems integrated for farm use could solve this issue.
Survey responses indicate that RMS observed in Italian farms has similar features to what is generally described in the literature (Metselaar et al. 2022): outbreaks occur more often in springtime with water temperature around 8-10° C and up to 15-16°C, with no substantial difference in water temperature between negative and positive farms. Contrary to what reported in Danish farms (Schmidt, Thompson, and Padros 2018), where the disease outbreaks are seen all year round, RMS in Italy is not seen during summer time, as expected due to high water temperature.
RMS outbreak most commonly affects 10-30% of units (45.1%) and up to 10% of the fish in these units (61.2%). Nevertheless, it is worth considering that these percentages may be underestimated, as small lesions can often pass unnoticed since RMS does not have any apparent impact on the growth rate, mortality, or feed conversion ratio. No geographical pattern for positive farms was found within each region or river basin area, where water was coming from the same water source or distributed within the same area.
RMS in Italian farms seems to follow a similar kinetic within the farms as reported in previous studies (Verner-Jeffreys et al. 2006). Once it is observed on a farm, fish will develop more severe clinical signs of RMS in approximately 3 to 4 weeks and resolves in further 8 to 12 weeks. It is a rolling infection, as during this period of about 3 to 4 months, some fish will start healing whereas others will start showing signs with long latency.
Oxytetracycline is the most common and effective antimicrobial used in the cases reported here. This is reported in early literature (Verner-Jeffreys et al. 2008) and confirmed in experimental studies (Schmidt, Henriksen, and Olesen 2021), but it is no longer favoured due to the long withdrawal period and the treatment related costs (Verner-Jeffreys and Taylor 2015). Interestingly, antibiotics use reported in previous questionnaire based studies (Schmidt, Thompson, and Padros 2018) is rare and observed as poorly effective.
It is a common practice to retain fish with RMS, as the condition spontaneously resolves, and there is no reported impact on either survival or growth. This disease management practice is more suitable for producers rearing large trout for the table or recreative fisheries, as opposed to portion table trout producers or organic farmers.
Furthermore, the survey shows that antibiotics are most commonly used when the disease affects more than 50% of the tanks, reflecting the relatively benign nature of the disease and the fact that treatment is used only when morbidity appears relevant.
Unusual findings in this study include that rainbow trout of less than 100g were relatively often affected, and that RMS is reported to recur in the same batch of fish. Only three recent descriptions of juvenile trout affected by RMS are available in the literature (Oh et al., 2019; Orioles, Galeotti, et al. 2022; Schmidt, Donati, and Lorenzen 2022), otherwise, the disease is reported to affect fish of more than 100g only.
Recurrence of clinical signs in the same fish is generally not considered possible, or at least very rare (Schmidt, Thompson, and Padros 2018), as it seems exposure of fish to RMS can trigger the development of natural immunity (Metselaar et al. 2022; Schmidt, Donati, and Lorenzen 2022). Also, it is difficult to follow the same batch of fish across all production cycles. This should be further investigated in future studies.
Regarding possible transmission routes, from the results of our study it is evident that veterinarians and farmers think water is the main source of infection, while the movement of eggs and fish within and across the farms are considered to have a lower potential impact on the transmission of the condition. Even though early studies on the subject reported that sites with very few suppliers of live fish and ova have a lower probability of infection due to their limited contact with other sites, we could not specifically investigate this risk factor here.
No other freshwater species were observed having RMS-like disease. It is worth noting that brown trout were also recently confirmed to be a susceptible species (Schmidt 2019; Scott 2022).
When considering biosecurity measures and predisposing factors for RMS outbreaks, no definitive conclusions can be drawn from the data gathered of this survey, but a few speculations are possible. Compared with previous surveys (Adam 2010; Schmidt, Thompson, and Padros 2018), in our study the number of fish deliveries to a farm or connections through the river network may not have any impact on the farms’ chances of developing the disease. Negative and positive farms were often both supplied by the same water source and none of the positive farms obtained fry from more than 4 different suppliers. This has been recognized as a risk factor (Adam 2010). None of the positive farms were based on closed systems, or using solely manual handling of fish; all positive farms and about 90% of negative farms used mechanical methods of handling fish. On the other hand, all small sized, closed farms that use mainly manual handling and hand netting were negative. As previously reported (Adam 2010), avoiding mechanical handling may represent a protective factor.
Stocks held on the same water supply were reported to develop the same conditions as in positive farms. This is compatible with what is reported in previous studies (Verner-Jeffreys et al. 2008).
Currently, there are no specific biosecurity measures for RMS, mainly due to the need for more knowledge about the biology of MLOs. Generally speaking, a closed farm where no fish are imported and movements of fish are restricted will be less likely to contract new diseases. This does not always seem the case for RMS as described in recent reports within an isolated RAS (Orioles, Saccà, et al. 2022). Horizontal transmission by direct or indirect contact is supported by cohabitation studies (Verner-Jeffreys et al. 2008; Jørgensen et al. 2019).
Vertical transmission could be possible as it was suggested that batches of eggs initially introduced the disease (Metselaar et al. 2022). Since the putative causative agent is likely an obligate intracellular bacterium, this could also indicate a higher risk of vertical transmission of the disease. It was not possible to investigate directly the role of eggs transfer inside or across different sites due to the lack of respondents’ collaboration and compliance. Eggs from the same supplier were used both in negative and positive farms, so the link was considered only circumstantial as reported in the literature (Verner-Jeffreys et al. 2008).
The role of vertical transmission needs to be investigated further, and the importance of this transmission route discussed in more detail with health managers and veterinarians. Another challenge is the presence of wild stock swimming freely in the waterbody.
Finally, no clear connection between the type and intensity of feeding and RMS was found.
In conclusion, RMS is still present in Italian rainbow trout farming, affecting about 30% of farms. Despite being widespread, the disease is not perceived as a major concern in the Italian trout farming industry, mainly due to its intermittent nature, low mortality and spontaneous resolution. However, after 20 years since the first observation in Europe (and even earlier considering Fleury, Vuillaume, and Sochon 1985), this survey reiterates some knowledge gaps about the disease, such as the biology, distribution, genomics, transmission routes, host specificity and pathogenicity of MLO. This has implications for biosecurity and management measures, as well as the impact of MLO in wild populations. Furthermore, we still need a reasonable estimate of the economic impact of RMS on the trout sector.