Introduction
Sea Star Wasting Disease (SSWD) refers to poorly described, non-specific clinical signs, including abnormal posture, epidermal ulceration, and limb autotomy (sloughing). It causes mortalities of over 20 species of sea stars and subsequent ecological shifts throughout the northeast Pacific (Work et al. 2021). It was initially thought to be caused by a densovirus (Hewson et al. 2014). The widespread presence of densoviruses in apparently healthy sea stars (Jackson et al. 2020) argues against this. One hypothesis suggests that SSWD is a sequela of microbial organic matter remineralization near respiratory surfaces, one consequence of which may be limited oxygen availability at the animals’ water surface (Aquino et al. 2021).
Asterias rubens is the most common and best-known starfish in the Northeast Atlantic and can reach a diameter of up to 52 cm, but usually between 10 and 30 cm. The colour of the starfish is variable but typically orange, pale brown or purple, whereas deep-sea specimens are pale. It has five broad, tapering arms at the base which are often bent slightly upwards at the tip when active (“The Marine Life Information Network: Common Starfish (Asterias Rubens),” n.d.).
This case report gives insights into a clinical case of SSWD in kept A. rubens, which occurred after ten new sea stars had arrived from a biological station in the North Sea in June 2021. It provides information about conducted treatments and clinical, microbiological, histological, and molecular biological examination results.
Materials and Methods
Sea star keeping facilities at Aquazoo Löbbecke Museum
Sea stars were kept in a public 2000 L marine community tank, representing the fauna of the North and Mediterranean Seas. A total of thirteen common sea stars lived together with two sand starfish (Astropecten irregularis), European plaice (Pleuronectes platessa), European flounder (Platichys flesus), common topknot (Zeugopterus punctatus), wide-eyed flounder (Bothus podas) and dream fish (Sarpa salpa). Sandy bottom substrate and several large live rocks provided many retreats. The fish and sea stars were fed mussels, fish, copepods, and lettuce. The water parameters were within normal ranges (temperature, pH, carbonate hardness, nitrate, phosphate, density).
The water parameters were measured once a week and were within the following ranges for the period June 2021 to January 2022: temperature: 14.4 - 15.9 °C, pH: 7.9 – 8.5, carbonate hardness (°dKH): 7 - 12, nitrate (mg L-1): 12 - 24, phosphate (mg L-1): 0.2 – 0.5, density (g cm-³): 1.0231-1.0249
Clinical and microbiological examinations of sea stars
Fresh preparations of affected sea stars were examined microscopically. Depending on the external macroscopic clinical appearance and the stage of the disease, the changes have been classified into different grades (Table 1). The affected tissue was sampled for microbiological examination using sterile swabs with transport media (Heinz Herenz, Hamburg, Germany) and sent to an external laboratory and isolated by culture (Chemical and Veterinary Investigation Office Ostwestfalen-Lippe (CVUA-OWL), Detmold, Germany). Additional resistance testing was ordered by determination of Minimum Inhibitory Concentration (MIC) in mg L-1 according to DIN EN ISO 20776.
Histological examination and PCR analysis for Sea Star associated Densovirus (SSaDV)
Samples of the ulcerated areas of the skin were fixed in 4% buffered formaldehyde for histopathology and embedded in paraffin. Three µm sections were cut and stained with hematoxylin and eosin (HE) and Giemsa by routine laboratory protocols (Romeis 1989).
To detect SSaDV, altered sea star tissue samples and unaltered tissue samples were sampled and stored in 100% isopropanol until examination. Genomic DNA was extracted after mechanical lysis in a QIAgen Tissuelyser II (Qiagen) using a commercially available DNA extraction kit in accordance with the manufacturer´s instructions (Qiagen DNeasy blood and tissue kit, Qiagen GmbH). After isolation, the DNA was measured using the NanoDrop 1000 spectrophotometer (NanoDrop Technologies), and samples were subsequently diluted to match the concentration of 50 ng mL-1. An endpoint PCR was performed with 0.2 U of hot-start KAPA 2G robust polymerase (PeqLab Biotechnologie GmbH), 1× KAPA A buffer, 200 nM of each primer, 200 µM of each dNTP, 5.0 μL of DNA samples and nuclease-free water to a final volume of 25 μL.
PCR was performed using the primers SSaDV_NS3_1_F and SSaDV_NS2_1_R_2 in a SensoQuest thermocycler (SensoQuest GmbH) with a PCR profile consisting of an initial denaturation step at 98°C for 30 s, 35 cycles at 98°C for 10 s, 64°C for 30 s, 72°C for 40 s and an extension step at 72°C for 2 min (Jackson et al. 2020). A negative control (Aqua dest.) was included in the analysis. All PCR products from affected or unaffected tissue samples and the negative control were applied to a 1% agarose gel with the addition of 4 µL Gel Red Nucleic Acid DNA marker (Biotium, Inc.) and 1 x TBE (Tris-Boric Acid-EDTA) buffer. DNA amplicons were subsequently separated in an electrical field. A DNA ladder (100 bp, Carl Roth GmbH) was used to determine the product size. The resulting bands were visualized under 302 nm UV light.
Treatment protocols
The starfish treated with marbofloxacin (Marbocyl® FD 1% 5mg kg-1, Vetoquinol, Ismaning, Germany) were injected intracoelomically three times every fourth day. The antibiotic was injected via a 27-gauge needle on the aboral surface at the base of a limb, near its junction with the central disc (Rosenberg et al. 2016).
The treatment in a trimethoprim/sulfonamide bath (Borgal® 24% 24mg L-1, Virbac, Carros, France) was conducted three times every third day for 3 h, respectively. For this purpose, a 5 L bucket was filled with one litre of tank water, and the antibiotic was added. The bucket was immersed halfway in the tank water and fixed to keep the temperature stable during the bath. Photos were taken of each diseased starfish’s oral and aboral side for individual identification. After each treatment, the animals were returned to the original tank.
Results
Clinical development of SSWD
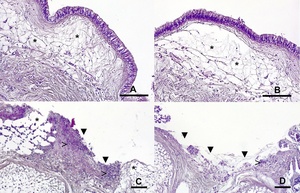
In early October 2021, the first common starfish was presented with an autotomy of one limb and eversion of viscera. Antibiotic therapy was initiated using marbofloxacin. Two days later, four more starfish showed early stages of ulcerative lesions (Figure 1), recognisable by white to light pink blurred roundish skin lesions. They also received marbofloxacin therapy and were returned to the tank. One sea star died on day 14 after the first injection of marbofloxacin. The other animals showed no significant improvements during the two-weeks treatment, but a slowly progressive deterioration of their condition (Figures 2, 3).
After the resistance test results were available and all other starfish also showed the described symptoms, all animals were treated in trimethoprim/sulfonamide baths. Symptoms did not improve during or after antibiotic treatment but progressively worsened. One starfish died fourteen days after the first bath with trimethoprim/sulfonamide. In the following ten weeks after the first symptoms appeared, individually severed arms were repeatedly recovered in the bottom substrate, which could no longer be assigned. Unfortunately, all remaining A. rubens starfish died in the end with a similar course of disease despite all treatment attempts. At the beginning of February 2022, the last tissue remains were found in the sand.
Clinical and microbiological examinations of Sea stars
Depending on the severity (low, medium, high), the animals had ulcerations of varying depth on different parts of the body surface, especially in the area of the arms. The blurred, roundish skin lesions were white to light pink. In the advanced stages of the disease (high grade), several severed arms could be found in the tank with signs of severe degradation (Figure 3).
Fresh preparations from the skin and viscera of the affected arms showed few marine epibiotic protozoa and a moderate infestation of bacteria which were identified by microbiological examination.
Microbiological examination of swab samples from the inside of the arm of the first diseased starfish revealed high concentrations of Vibrio sp. and Delftia sp. Only Delftia sp. could be further cultured to perform a resistance test. Vibrio sp. was no longer culturable for resistance determination. According to the antibiogram, only the active substances chloramphenicol and the combination trimethoprim/sulfonamide were sensitive (Table 2). Subsequent microbiological examinations from the vicinity of ulcerated skin sites did not reveal any other bacteria.
Histological examination and PCR analysis for Sea Star associated Densovirus (SSaDV)
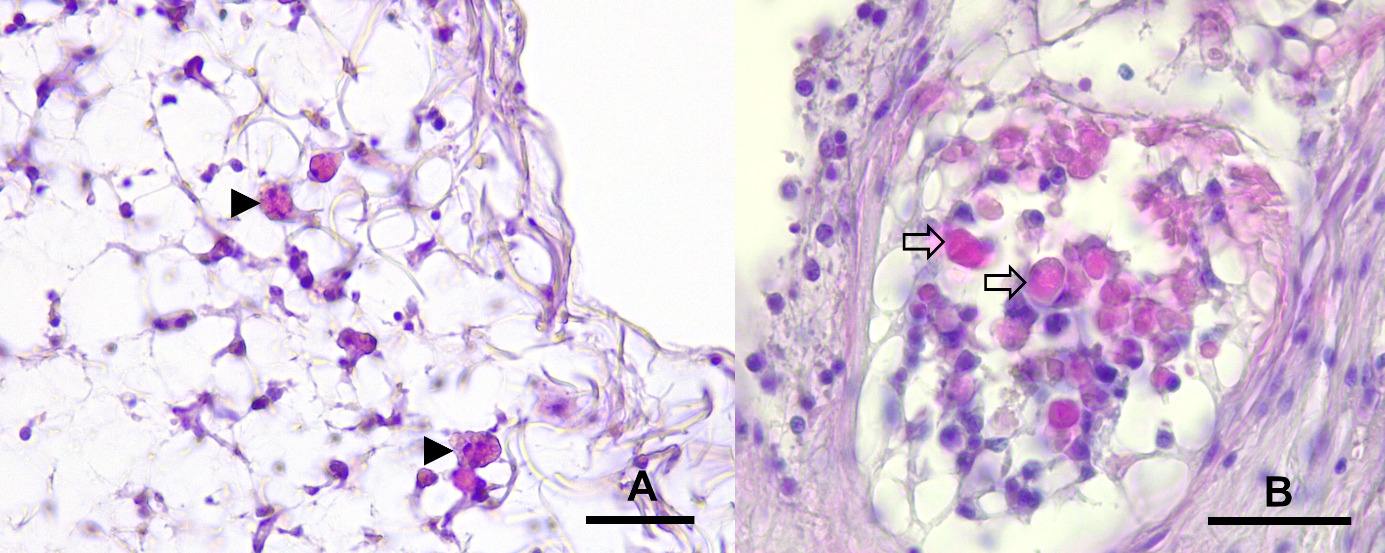
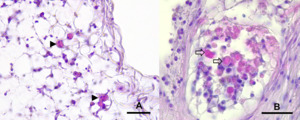
Histological examinations showed that the alterations were mainly located in dorsal and lateral areas near the spines of the examined arms of A. rubens. Macroscopically altered areas showed several degrees of alteration with cleft formation between epithelium and the underlying connective tissue, a loss of epithelium as well as severe infiltration with haemocytes and low amounts of eosinophilic granular cells (EGC) in superficial areas. In macroscopically unchanged areas, subepithelial layers were severely widened, and oedema was suspected (Figure 4). Furthermore, spines of macroscopically unaltered skin areas showed a beginning degeneration of the ossicle structure. Ossicles showed different stages of degeneration with severe necrosis in superficial areas and, in deeper layers the beginning of degeneration of trabeculae with an increase of cellular density and accumulation of proteinaceous debris as well as infiltration with eosinophilic granular cells (EGC) (Figures 5, 6).
None of the PCR products from endpoint PCR showed a positive band in gel electrophoresis. No sequences of SSaDV could be amplified in affected or unaffected tissue samples.
Discussion
The present case report describes the course of an acute Sea Star Wasting Disease (SSWD) in captive A. rubens. Taken measures of treatment, unfortunately, did not have the expected effect, and examinations did not conclusively clarify the primary causes for the onset of the disease.
Previous reports about Sea Star Wasting Disease described Sea Star associated Densovirus as a primary pathogen (Hewson et al. 2014). To date, this theory was not verified by multiple studies. Instead, these studies argued that a profound microbiome disturbance probably induces this disease (Aquino et al. 2021; Lloyd and Pespeni 2018).
In accordance with these findings, our results showed that endpoint PCR targeting a gene sequence from SSaDV was negative. Therefore, we exclude the involvement of this densovirus. Furthermore, the diseased Sea Stars came from an area in the North Sea where up until now, no densovirus infections have been described in Sea Stars. Nevertheless, it must be considered that a positive control could not be included in the examination, and therefore a false negative result cannot be entirely excluded.
Histological and microbiological examinations point towards chronic inflammation with the participation of bacteria. It could be shown that the lesions deeply infiltrated the body wall of A. rubens and severely affected ossicle integrity. The bacterial colonisation could be regarded as a primary or secondary infection; in this respect, a definitive cause of disease could not be proved. Histologically, the presence of such bacteria could not be definitely proved. Yet, microbiological examination revealed a profound infestation of altered tissue areas with Vibrio sp. and Delftia sp.. Usually, the isolation of unspecific, ubiquitously occurring bacteria in skin wounds additionally underline the theory of the involvement of copiotroph bacteria that degrade organic matter (Aquino et al. 2021; Oulhen et al. 2022). It furthermore indicates a possibly weakened immune system of affected animals, as opportunistic bacterial pathogens usually cause infection in weakened animals and mostly act as secondary pathogens (Roberts 2012). Therapy attempts using antimicrobial substances (marbofloxacin, trimethoprim/sulfonamide) had no positive effect on the animals. It was previously reported that the administration of enrofloxacin by injection also did not significantly alter mortality rates in captive starfish affected by SSWD (Wahlstrom et al. 2015).
In this case, only A. rubens with a rough skin surface were affected. The two A. irregularis with their smooth skin surface did not show any abnormalities, even though these animals were kept in the same tank. Also, histological examination indicated that spines could be the starting point for SSWD, as spines in macroscopically unaltered skin areas showed initial degradation. A previous study showed that the susceptibility to SSWS was significantly and positively correlated with surface roughness, a key determinant of diffuse boundary layer thickness (Aquino et al. 2021). An unexpected and notable occurrence during another study period was the presence of pedicellariae on the external surface of Dermasterias imbricata. Pedicellariae are small pincer-shaped defence organs on the outer body wall surface (Blowes et al. 2017). It was previously observed that the removal of pedicellariae from D. imbricata and complete separation from Asteriidae sea stars resulted in the disappearance of clinical symptoms of SSWD in affected animals (Wahlstrom et al. 2015). Nevertheless, we could not detect any alien pedicellariae on the surface of our common starfish. As no quarantine tank with the appropriate temperature was available at the time of the disease outbreak, separation from the sand starfish was impossible.
A proliferation of copiotrophs prior to the onset of SSWD, followed by the appearance of putative facultative and strictly anaerobic taxa at the time of lesion formation, was previously described. SSWD lesions were induced in Pisaster ochraceus by enrichment with various organic matter sources (Aquino et al. 2021). According to the authors, oxygen deficiency at the animal-water interface may be caused by heterotrophic microbial activity in response to organic matter loading. SSWD was also triggered by moderately (∼39%) depleted oxygen conditions in aquaria, suggesting that small perturbations in dissolved oxygen may exacerbate the condition (Aquino et al. 2021). In accordance with this, our new starfish were exposed to elevated levels of organic matter and high levels of bacteria in the water during transport. They were transferred to a well-stocked aquarium after acclimatisation.
A temporary stress situation due to transport, transfer, and acclimatisation to new conditions is quite understandable and usually not avoidable. Nevertheless, such high mortality, as in this case, is unusual. Still, this might be one factor adding to the development of SSWD in these animals.
In the final stage, all starfish succumbed to limb autotomy. The survivors showed blotchy colouration and a weakened appearance. Despite therapy with antibiotics, none of the thirteen A. rubens survived after this outbreak of SSWD.
Conclusion
This report reflects a disease event that affected all A. rubens starfish in an aquarium within a few weeks. The goal of the therapy was to improve the condition of all animals. Our here-gained experience with SSWD and the latest research results suggest that newly arriving starfish should be transferred to a quarantine aquarium separated by species. Especially for starfish species with rough surfaces, regular measurement of dissolved oxygen and reduction of organic water pollution seems advisable.
The administration of antibiotics did not significantly alter mortality rates in captive sea stars affected by SSWD. Therefore, it cannot be recommended to treat this disease in commonly affected species. As SSaDV, obligate pathogenic bacteria or parasites were not detected, this case report underlines the theory that environmental influences probably cause SSWD. Multiple influencing factors such as stress, water quality, organic load, or lowered immune system status as well as a rough surface structure of the animals might be involved in disease development.


_of_sswd.jpeg)
_of_sswd.jpeg)


_of_sswd.jpeg)
_of_sswd.jpeg)