1. Introduction
Atlantic mackerel (Scomber scombrus) is present in the North Sea. “Northeast Atlantic (NEA) mackerel” is a term used to define the population of mackerel present in the extended area from the northern Norwegian Sea, to the Iberian Peninsula and from Iceland to the western Baltic Sea (ICES 2021). Mackerel are a migratory fish which start spawning from January to February in the Iberian Peninsula waters and complete spawning in July in northwest Scotland and the North Sea (ICES 2021).
Mackerel is a very important and valuable pelagic fish in European waters (Rybicki et al. 2020), and the same is true in Scotland. According to Rybicki et al. 2020, mackerel accounts for 32% of pelagic EU fleet total value (average of 2013-2017). This species is abundant year-long in Scotland and provides a popular sport for recreational anglers during the summer months. In terms of capture fisheries, it is the most valuable species landed by the Scottish fleet and accounts for 37% of the value of landings for Scottish vessels (Marine Scotland 2021).
Myxozoan parasites belonging to the order Multivalvulida of genus Kudoa have been linked to post-mortem myoliquefaction of fish muscles in several host fish orders (e.g. Japanese anchovy (Engraulis japonicus), lumpfish (Cyclopterus lumpus), silver scabbardfish (beltfish) (Lepidopus caudatus), Pacific hake (Merluccius productus), cape dory (Zeus capensis)) (Henning, Hoffman, and Manley 2013; Kristmundsson and Freeman 2014) in different geographical regions with a broad range of environments such as South Africa, Australia, the west coast of North America, the Japanese sea and the North sea (Whipps and Kent 2006; Henning, Hoffman, and Manley 2013; Højgaard, Homrum, and Salter 2022). According to Whipps and Kent 2006, and Henning, Hoffman, and Manley 2013, the species Kudoa thyrsites had been found in 37 different fish species of marine teleosts of 18 different families including Clupeidae, Merlucciidae, Salmonidae, Scombridae and Trichiuridae across nine fish orders. This condition is colloquially referred to as ‘soft tissue’, ‘milky flesh’ or ‘jelly flesh’ (Henning, Hoffman, and Manley 2013) characterised by an unpleasant appearance and soft, or viscous texture of the flesh commonly seen in areas of the trunk muscle (Levsen, Jørgensen, and Mo 2008). This phenomenon has been well studied due to the economic impact on the fisheries and aquaculture industries (Lafferty et al. 2015), in farm-reared Atlantic salmon (Salmo salar) (Braden et al. 2018; Jones and Long 2019) and Pacific hake (Merluccius productus) (King et al. 2012) for instance.
K. thyrsites is a species well known to cause soft tissue. The parasite cycle is not well understood but it has been hypothesised that K. thyrsites has a complex cycle with more than one host (Braden et al. 2018; Jones and Long 2019). The plasmodia infections are seen as pseudocysts in myofibrils of the somatic muscle and occasionally seen in the cardiac muscle with no clinical signs observed (Whipps and Kent 2006). These parasite infections do not cause mortality of the host, however after host death, within approximately 38 to 56 h, proteolytic enzymes are released by the parasite in the host. The enzymatic effect is only apparent after the death of the host. The degree of musculature degradation seems to be influenced by the severity of infection, storage temperature and quality of the muscle (Henning, Hoffman, and Manley 2013; Bolin et al. 2021). Giulietti, Nedberg, et al. (2022) also found an association between the dispersion of free myxospores and the level of myoliquefaction of the host fish muscle. The condition results in unmarketable fillets resulting in substantial economic losses in fisheries and aquaculture industries worldwide and a reduction in consumer confidence (Whipps and Kent 2006; Henning, Hoffman, and Manley 2013; Lafferty et al. 2015; Braden et al. 2018; Bolin et al. 2021; Giulietti, Nedberg, et al. 2022).
In the North Sea, soft tissue has also been reported in mackerel with the etiological agent known for some time. Levsen, Jørgensen, and Mo (2008), stated that it was first reported in Norway in 1968, and Whipps and Kent (2006), reported the presence of the parasite in England. There are sporadic observations of this parasite in Atlantic salmon from Ireland (Palmer 1994). Recently, Giulietti, Karlsbakk, et al. (2022) stated that in Norway the occurrence of soft tissue in mackerel seemed to be steady until 2019, whereas in 2020, the occurrence of this condition increased by three- to six-fold. The authors also stated that this condition appears to affect mainly the most commercially valuable size group, fish of over 400 g, and therefore the fisheries industry may be affected.
Mackerel caught in the North Sea and frozen fresh at sea were defrosted for processing at a fish processing plant located in Fraserburgh, in Aberdeenshire. After defrosting, some fish displayed soft tissue which was described by the processer as having ‘turned to mush’ (Figure 1a and 1b). This has been an ongoing observation for some time and although it is not observed in a large number, it can occasionally be up to 60 kg of fish, which is a very small proportion of the total of fish processed. The soft tissue is unusual and raised concerns.
Although K. thyrsites has been reported in mackerel from North Atlantic waters adjacent to the Scottish Zone ‘exclusive economic zone’ (EEZ) (Figure 1c) for many years, to date, there have been no official reports of the parasite in Scottish waters. The parasite is anecdotally known to be found in mackerel in the area, as Scotland is part of the migratory areas of mackerel (ICES 2021), however, the level of infection in Scottish waters is unknown. This case study aims to report the presence of K. thyrsites-induced post-mortem myoliquefaction in mackerel caught in Scottish waters.
2. Material and methods
2.1. Fish samples
Four commercial sized frozen mackerel were received for diagnostic testing in November 2021 from a fish processing plant in Fraserburgh, Aberdeenshire. As the mackerel had been dead for a few days and defrosted for 48 h at the processing plant, only a section of skin and skeletal muscle from two fish were used for histopathology. Tissue was fixed in 10% neutral buffered formalin (NBF). Additional samples of muscle tissue from all four fish were also taken at four different areas across the lateral flesh and pooled into a tube of 100% ethanol for molecular genetic analysis.
2.2. Histopathology
After 24 h in NBF, fixed tissue was routinely processed for light microscopy in an Excelsior AS tissue processor (Epredia) and embedded in paraffin wax (Cellpath). Sections of 3 and 5µm thickness were cut using a rotary microtome (Leica biosystems), and stained with Harris Haematoxylin and Eosin (H&E) and May Grünwald Giemsa (MGG) using standard protocols. Slides were examined by light microscopy using a BX50 microscope (Olympus) and photomicrographs prepared using a Slideview VS200 slide scanner (Olympus).
2.2. Molecular testing
DNA was extracted from 10 mg of muscle tissue using the DNEasy Blood and Tissue kit (Qiagen) on the QIAcube Biorobot (Qiagen) under the manufacturer’s standard extraction protocols. Amplification of a 890bp product of the small subunit rDNA region was performed using 1F (5’‑CTATCAACTAGTTGGTGA-3’) and 2R (5’‑CAATGTCTGGACCTGGTG-3’) primers from the assay described by Hervio et al. (1997), using the cycling conditions detailed in the paper. PCR amplification was performed as a 40µL reaction containing 28 pmol of each primer, 10 mM dNTPs, PCR buffers and 1.6 U Taq polymerase (Bioline). DNA sequencing was performed by DNA Sequencing & Services (MRC I PPU, School of Life Sciences, University of Dundee, Scotland, www.dnaseq.co.uk) using Applied Biosystems Big-Dye Ver 3.1 chemistry on an Applied Biosystems model 3730 automated capillary DNA sequencer. Resulting data sequences were reviewed using Sequencher software (Gene Codes) and sequence identity was determined using the Basic Local Alignment Search Tool (BLAST) (National Centre for Biotechnology Information; Altschul et al. 1990).
3. Results
3.3. Gross pathology
All four mackerel displayed clear signs of soft tissue along the body (Figure 1a). The skin was cut open along the body and the entire musculature of the four fish was found to be liquefied, with a greyish appearance (Figure 1b).
3.4. Histopathology
Microscopically, the red skeletal muscle appeared normal and the white skeletal muscle exhibited large areas of fibre necrosis and many Myxosporea spores were observed in the interstitial space between the fibres and among the necrotic liquefied muscular fibres (Figure 2a) in both fish. When viewed apically (Figure 2b-d), the spores consisted of four stellate shell valves with four pyriform nematocysts (polar capsules) with one noticeably largest always opposite the smallest. The mid-sized polar capsules appeared to touch, with the large and small polar capsules adjacent to this interface. In lateral view, polar capsules were arranged pyramidally with the narrow ends at the apex. This is consistent with the myxospores of K. thyrsites (Kabata and Whitaker 1981; Herrell and Scott 1985).
3.5. Molecular results
The presence of the Kudoa species was confirmed by polymerase chain reaction (PCR) and then confirmed by a partial DNA sequence analysis. A PCR amplification product of the expected size (890 bp) were seen on the gel in all four mackerel samples submitted for pathological examination.
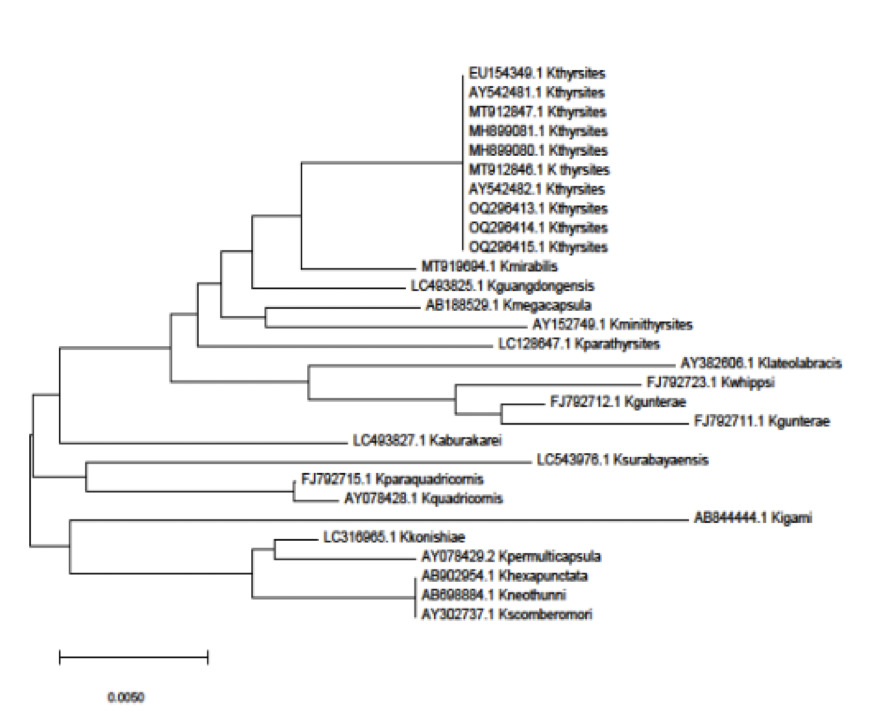
A partial sequence of DNA extracted from three mackerel muscles showed 100% identity with Kudoa thyrsites, accession number MH899081.1. These were submitted to Genbank under accession numbers (OQ296413, OQ296414, OQ296415) (Figure 3). The fourth did not produce sufficient high quality sequence for inclusion in the analysis.
4. Discussion
Mackerel is the most valuable pelagic species in Scottish fisheries. This species is known to be one of the hosts of K. thyrsites, the causative agent of the myoliquefaction condition ‘soft tissue’. This condition is known to have caused substantial financial losses in Canadian farmed salmon (Funk et al. 2007; Jones et al. 2016) but the real economic and financial impact of this parasite on the mackerel in Scottish fisheries is unknown. To date, there is little information about this parasite in fish living in Scottish waters.
In Norway, the parasite was also detected in mackerel, and it seems to show 100% identity between the two strains based on the rDNA gene region sequenced to the one found in the Scottish mackerel. Giulietti, Karlsbakk, et al. (2022) stated that in the northeast sea, the soft tissue condition seemed steady for over a decade and in 2019 and 2020, there was an increase in the occurrence of this condition. Levsen, Jørgensen, and Mo (2008) and Giulietti, Karlsbakk, et al. (2022) also stated soft tissue seems to affect the most valuable sized group of mackerel, those over 400 g. This condition seems to develop in individuals with high parasite density in the musculature. In Norway, it was found that the prevalence of the parasite in mackerel is much higher than the development of this condition. This evidence is of great importance as Scotland is part of the migratory areas of mackerel (ICES 2021) and the parasite characteristics may have an identical pattern. Levsen, Jørgensen, and Mo (2008) and Giulietti, Karlsbakk, et al. (2022) speculated that a potential source of parasite infection might be in the western and southern spawning areas which overlap with the potentially contagious areas of K. thyrsites (Iberian Atlantic coast, southern Ireland, and England). In addition, body size-weight and sex appear to be the most important factors for the infections in mackerel (Giulietti, Karlsbakk, et al. 2022).
Another potential factor could be environmental conditions. Santos et al. (2019) have found a seasonality pattern of Kudoa sp. infection associated with salinity levels. Moran, Kent, and Whitaker (1999) also found seasonality to influence the infective stage of Kudoa thyrsites via natural exposure of farmed Atlantic salmon in British Colombia, Canada. However, little is known as there are not many studies investigating the effect of environmental conditions (e.g., temperature and salinity) on the occurrence of the condition myoliquefaction and Kudoa sp. infections (Bolin et al. 2021).
In summary, K. thyrsites is known to be found in mackerel in Scotland but little is known about the K. thyrsites in Scottish waters and the economic impact of the ‘soft tissue’ condition. This case study is the first official report describing the identification of K. thyrsites associated with liquefied tissue in mackerel. This study is a preliminary report and it would be valuable to investigate the prevalence of the parasite in the mackerel caught in the Scottish waters and the occurrence of soft tissue.

_atlantic_mackerel_exhibiting_soft_tissue_when_pressed_by_a_finger__b)_fillets_of_macker.png)
_*kudoa*_myxospores_in_the__interstitial_(https___www.sciencedirect.com_topics_earth-and.png)

_atlantic_mackerel_exhibiting_soft_tissue_when_pressed_by_a_finger__b)_fillets_of_macker.png)
_*kudoa*_myxospores_in_the__interstitial_(https___www.sciencedirect.com_topics_earth-and.png)