Introduction
Currently, aquaculture (cultivation of aquatic organisms), produces more than 53 million tons of seafood, worth US$155 billion in 2020 (FAO 2022). Nile tilapia (Oreochromis niloticus L.), is being farmed in more than 135 countries. Major producers are developing countries, including China, Indonesia, Philippines, Thailand, Egypt, Honduras, Ecuador. The global Nile tilapia production increased rapidly, and was 5.6 million tons in 2020, with a market value of US$11.2 billion (FAO, 2022).
Nonetheless, the intensification in tilapia culture has led to disease outbreaks with significant losses in production (Brum et al. 2017). Haemorrhagic septicaemia or motile Aeromonas septicaemia (MAS) is caused by the bacterial species Aeromonas hydrophila and is the most common bacterial disease that infects wild and cultured tilapia (Austin and Austin 2007). Massive losses of tilapia due to Aeromonas infections have been reported worldwide. A. hydrophila is an opportunistic pathogen that results in high economic losses and can infect not only fish, amphibians and reptiles but also mammals, including humans (Plumb and Hanson 2011; Stratev et al. 2015). To avoid economic losses related to chemical and biological hazards, several veterinary drugs are used in aquaculture to prevent or treat disease outbreaks (Rico et al. 2013; Reverter et al. 2014).
However, the use of veterinary drugs is becoming increasingly restricted because they present adverse effects on the environment and both animal and human health. For example, massive use and misuse of antibiotics have resulted in the selection and emergence of resistant bacteria strains (Seyfried et al., 2010, Brunton et al. 2019) or the presence of antibiotic residue in the muscle of commercialized fish, thus having potential consequences on human health (Sarter et al. 2007; Alam and Haque 2021). Antimicrobial resistance (AMR) is recognized as a major global threat to ecosystem health including animal, human, plant and environmental health, And a recent meta-analysis has shown that aquaculture environments are reservoirs of high levels of resistance and multi-resistance throughout the world (Reverter et al. 2020). Several tropical areas, particularly in the Mekong Delta, are strongly exposed to AMR risks in aquatic environments (Schar et al. 2021). Due to the positive correlation between temperature and mortality of infected fish, fish diseases are expected to increase with global warming (Reverter et al. 2020). AMR in aquatic environments is thus considered an urgent issue of high concern.
In this context, preventive and sustainable approaches are recommended for disease management, and the use of plant-based products in fish farming not only as chemotherapeutics but also as feed additives is widely recognized as a valuable resource (Citarasu, 2010; Wang et al. 2015), Many types of biological activity have been recorded for medicinal plants, including growth promotion, appetite stimulation, immune stimulation as well as antimicrobial and antistress properties (Citarasu, 2010; Chakraborty and Hancz 2011). The use of herbs as feed supplements can be very beneficial in improving fish growth and feed efficiency, as well as contributing to better disease prevention (Reverter et al. 2021), thereby enhancing sustainability of the aquaculture value chain.
Aiming to study alternatives to antibiotics, we selected and tested four plants present in the tropical regions — Schinus terenbinthifolius, Murraya koenigii, Aphloia theiformis and Pelargonium roseum —to prevent or mitigate epizootic diseases in fish caused by A. hydrophila. These plants are common in tropical areas, and are not threatened by extinction, but very few studies have reported on their pharmacological activities and, to our knowledge, none have been reported on O. niloticus using these dry plants.
Known as pink pepper or Brazilian pepper, S. terebinthifolius has potent antioxidant and antibacterial activities against the growth of Staphylococcus aureus and Pseudomonas aeruginosa with minimum inhibitory concentrations (MIC) of 16 μg/mL and 32 μg/mL (Salem et al. 2018). Its essential oil is effective against Gram-positive bacteria however, Gram-negative bacteria are not inhibited, but their growth is significantly reduced by the essential oil (Dannenberg et al. 2019). To our knowledge, only one article has reported its usage on ornamental fish (Porto et al. 2020)
M. koenigii, family Rutaceae, is commonly known as the curry-leaf tree. It is a native of India, Sri Lanka and other South Asian countries. Many ethnobotanical and pharmacological properties have been recognized in this plant along with antimicrobial properties (Handral, Pandith, and Shruthi 2012). Against fish pathogens, Wei et al. (2008) showed that ethanol or methanol extract of M. koenegii leaves has antibacterial activity against A. hydrophila and other fish bacteria. In vitro experiments have shown that two compounds isolated from its ethyl acetate extract, namely isomahanine and mahanine, have antibacterial activity on Flavobacterium columnare and Streptococcus iniae, both major pathogens for tilapia culture (Meepagala, Schrader, and Burandt 2013).
A. theiformis is a tree or shrub belonging to the family Aphloiaceae. It is a widely distributed species occurring in West Africa, South Africa, Madagascar, Comoros, Mascarene Islands and Seychelles. This plant is listed in the French Pharmacopoeia and in the Reunion Island and Mauritius, it is traditionally used against various disorders (diarrhoea, jaundice, fever) and it has antibacterial activity, even against Gram-negative bacteria (Jelager, Gurib-Fakim, and Adsersen 1998).
P. roseum is a species belonging to the Geraniaceae family. This species indigenous to southern Africa is now widespread and has also been introduced in Mediterranean Europe. Antibacterial activity of the essential oil of this plant has been demonstrated (Carmen and Hancu 2014), as well as efficacy against veterinarian ectoparasites (Ellse and Wall 2014). Exposure to the extract of P. roseum in water can reduce the microbial load of skin and gills in carp (Mohamadi, Zamini, and Vahabzadeh 2013) and it is an effective anaesthetic on two cichlid species (Can et al. 2018). Bioactive plant molecules can also be antinutritional in fish, decreasing the efficient utilization of feed nutrients. Therefore, their effect on fish physiology and the adequate dosage needs to be studied (Krogdahl et al., 2010).
Here, we investigated the effects of dietary supplementation with these four plants on fish (Oreochromis niloticus) mortality induced by experimental infection with A. hydrophila. We also measured the effects of an enriched diet using raw dry plants on the zootechnical and growth performances of fish (feed conversion ratio, specific growth rate and condition factor).
Materials and Methods
Plant and feed preparation
Leaves of P. roseum, M. koenigii, A. theiformis and drupes of S. terenbinthifolius were obtained from Reunion Island (Aplamedom). Leaves and drupes were dried in an oven at 50ºC, ground and sieved at 250 µm. Supplemented feeds were prepared using powdered commercial feed dampened with water, and plant powder was added at a ratio of 4% (40 g/kg of feed). This mixture was re-pelleted into pellets of ≤450 µm diameter for the first 20 days of feeding and of 725 µm diameter for the remainder of the experiment. Control feed was prepared as above without any plant powder. Fish were fed twice a day at a rate of 5% of their live body weight. During the trial, the amount of feed intake for each treatment was adjusted according to the biomass.
Fish and experimental facilities
O. niloticus juveniles, both male and female (2.91 ± 0.29g), were randomly distributed into five glass aquaria of 290 L (n=120 fish) in a recirculating aquaculture system (RAS) with a mechanical and biological filter. Each tank also had an individual mechanical filtration system and an air stone linked to a central air compressor to maintain continuous aeration. To prevent cross-contamination due to feed, each aquarium was isolated from the RAS during feeding time (around 1 h). Each aquarium was cleaned of fish faeces by siphoning and about 20% of the water was renewed daily. Filter foams were cleaned every two days. We used a 12:12 h light-dark cycle. Water parameters during the experiment were as follows: temperature: 27.48 ± 0.91ºC; pH: 7.84 ± 0.21; NO2: 0.00 – 1.05 ppm; NO3: 5.02 ± 2.89 ppm.
Growth performance and feed utilization
All procedures involving fish were performed on anaesthetized fish. Fish were anaesthetized using eugenol at 50 µL/L. The weight and length of fish were measured at 0 days, 12 days, 27 days and 40 days of the experiment. Condition factor (CF), feed conversion ratio (FCR) and specific growth rate (SGR) were calculated from the following equations:
CF=[Weight(g)/Length(cm)3]×100;
FCR=total feed intake (g)/total wet weight gain (g);
SGR=ln final mean body weight−ln initial mean body weighttime interval (days)×100.
Bacteria and median lethal dose (LD50)
A strain of A. hydrophila, isolated from a diseased fish (Clarias gariepinus) from Indonesia was selected for this study after previous comparative testing with other Aereomonadeceae strains on O. niloticus juveniles. This strain preserved in glycerol was revived three times on TSA agar (24 h at 30ºC) and then some colonies were picked and incubated for 24 h at 30ºC in a TSB liquid broth medium before being used for DL 50 and disease challenge.
Bacterial concentration was verified using direct counts of colony forming units (CFU) of solution cultured for 24 h in Petri plates. To determine the bacterial dose to be injected into fish, we carried out an LD50 trial. Sixty fish of 12.1 ± 0.3 g were divided into six groups (n=10) and each group was placed in an isolated aquarium (30 L). Anaesthetized fish received an intramuscular injection on the left side of the body. Each group was injected with a serial dilution of an A. hydrophila dose; the dilution fold was 1/3 for each dilution step. Subsequently, mortality was recorded daily until no additional mortality was observed and the median lethal dose (LD50) was calculated using the Reed–Muench method as follows:
Proportionate distance=% mortality at dose next above 50%−50%% mortality next above 50%−% mortality next below 50%
and the concentration 5.12 x 104 CFU/mL was determined as LD50. However, at the end of the feeding period, the size of fish was expectedly higher than those of fish used for LD50 determination and that concentration therefore was unable to elicit infection in fish in a preliminary test before the Disease challenge test. Because LD50 is size-dependent and considering that fish used for the disease challenge were much heavier (+348% in weight) than those used in the LD50 test, the bacterial concentration for the bacterial challenge was increased to 0.1 mL of 6.36 x 105 CFU/mL. This dose was used to infect fish for the experiment.
Disease challenge test
After 40 days of feeding, 45-60 fish from each different treatment and control group were randomly sampled, anaesthetized and then injected IM on the left flank with 0.1 mL of A. hydrophila. Following injection, 20 fish from each treatment were placed in glass aquaria (30 L) equipped with an individual mechanic filtration system and air bubble stone (Three aquaria per treatment). Fish were no longer fed. Mortality was recorded daily until no additional mortality was observed. The trial was terminated 8 days after the injection.
Statistical analysis
Differences between treatments for weight, length and CF were analysed using one-way Kruskal-Wallis tests (one-way analysis of variance (ANOVA) on ranks) because assumptions of normality (Shapiro–Wilk test) and variance test (Fisher test) were not met. A chi-square analysis was used to compare the mortality rates among the experimental treatments and control.
The relative per cent of survival (RPS) was determined using the following formula:
RPS=[1−mortality in the treated groupmortality in the control group]×100
Results
Growth performance and feed utilization
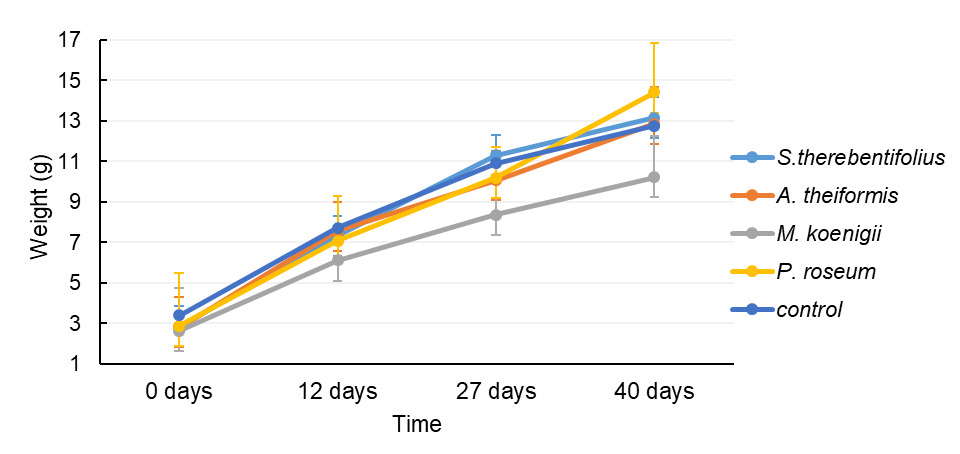
No significant differences were found in fish weight after 12; 27 and 40 days (one- Kruskal-Wallis; p=0.26, p=0.14, p= 0.15, respectively) between plant treatments. The mean weight for each plant treatment and control is shown in Figure 1. There were no differences also in total fish length of fish (Kruskal-Wallis p=0.90, p= 0.32, p=0.2, respectively).
The CF observed during the experimentation was not significantly different between plants treatments (Kruskal–Wallis test, P=0.058 Except for M. koenigii, the feed efficiency (FCR) observed with the plant treatments was lower than that observed in the control group growth (SGR) which was higher for all plant treatments than in the control group (Table 1).
Experimental infection
Fish infected with A. hydrophila started to die 19 h after contamination. ‘Fin rot’ or ‘skin rot’ was observed and infected fish usually showed dark pigmentation, exhibited slow movements (lethargy) and developed ulcers and exophthalmia. Other symptoms included the presence of several focal haemorrhagic necroses in skeletal muscles and over the visceral and peritoneal surfaces.
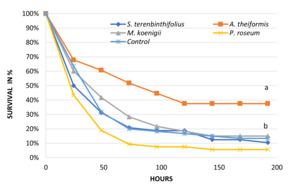
Survival after eight days of infection is shown in Figure 2. Mortality rates and RPS varied with the plant powder in the feed (Table 2). Mortalities were significantly different between treatments (chi-square, 22.90, d.f. 4, p<0.001). Fish fed with A. theiformis showed significantly lower mortality than all other treatments and control (6.84, d.f. X, p<0.009) (Figure 2 and Table 2).
Discussion
Carefully applied plant-based methods used for the prevention and treatment of various fish illnesses do not appear to entail any risks of polluting the environment, or damage to fish, other animals, or humans. Although medicinal plants are natural products, they can induce physiological changes in fish, improve their fitness and enhance their resistance to pathogens (Reverter et al. 2014; Awad and Awaad, 2017). However, careful consideration should be given to the use of some plants on aquatic animals as they can be very active or even toxic for them. The mode of action of the selected plants and their derivatives are attributed to the presence of many active ingredients, such as alkaloids, steroids, phenolics, tannins, terpenoids, saponins, glycosides and flavonoids (Harikrishnan, Balasundaram, and Heo 2011).
Although the concentration of active substances is highest in alcohol extracts or essential oils, the use of powdered plants is the simplest method for administering these plants to fish in feed.
Reverter et al. (2020) demonstrated that the efficiency of herbal medicine depends more on the right dosage than the form of the plant used and that powdered plants, extracts and essential oils are equally effective when used at the appropriate dosage. Oral administration seems to be the most appropriate way to use plants as therapeutic agents for prophylaxis and metaphylaxis in aquaculture. Through feed, handling stress is avoided and reduces workload of fishfarmer. Furthermore, compared to other forms of herbal compounds (extracts, essential oil), powdered plants have several advantages: they require little technical knowledge to be added to fish feed, are more stable, readily available and cost less, generally making them more affordable, particularly for small farmers.
In our study, growth performance (SGR) was higher than the control for all plant treatments, with SGR showing rates 3% to 21% higher than that of the control; likewise, feed utilization (FCR) was better than the control for all plant treatments except M. koenigii. Further studies are needed to investigate the effect of these plants as dietary supplements to stimulate tilapia growth, but the results observed here with P. roseum are noteworthy
Only one study (Al-Sagheer et al., 2017) has reported positive effects on growth in Nile tilapia for P. roseum essential oil extracts. Harikrishnan, Balasundaram, and Heo (2011) suggest that the essential oil extracts affect the development of indigenous intestinal flora, but the exact mechanism of action of plants in the metabolism of fish is still unclear. Our findings showed positive results against Aeromonas septicaemia in fish fed with A. theiformis. To our knowledge, there are no reports regarding A. theiformis used in tilapia or in other fish species. The major compounds contained in the leaves of A. theiformis are flavonoids and tannins (Hsoidrou et al., 2014). Numerous studies have reported the role of flavonoids in immunostimulatory and anti-inflammatory activity and the leaves of A. theiformis are also known to be rich in mangiferin (Danthu et al. 2010), a xanthone with an immune-stimulant effect (Muruganandan, Lal, and Gupta 2005). These compounds may explain the better survival of fish fed with this plant powder after a challenge with a bacterial infection. The efficiency of plants depends on the dose, duration time and the route of administration. However, the dose is still the most important factor because it varies with the plant species and form used (Harikrishnan, Balasundaram, and Heo 2011). Based on the scooping review, Reverter et al. (2020) point out that the posology of dry plants in aquaculture is very variable (0.1 - 420 mg/100 g fish*day). Taking into account the percentage of dried plants and the feeding rate, the posology used in this study, 50 mg/100 g, is well within the range. We suggest that in an exploratory screening such as the present study, a relatively low level of incorporation of dry plants should be maintained in order to avoid any possible adverse effects. Treatment duration is another important factor to consider for economic viability. Four weeks seems to be the most common duration reported in the literature and short periods of between 2 and 4 weeks are as effective as longer ones (≥8 weeks) (Reverter et al. 2020). Thus it could be possible shortening time of administration but further research is needed to test different feeding durations and doses to optimize the treatment with A. theiformis. Our findings suggest that the addition of 4% of powdered A. theiformis leaves in the fish feed may improve the resistance of fish against A. hydrophila infection without impairing growth performance,hence, this plant seems to be a promising dietary supplement to prevent diseases in Nile tilapia. Bioactive plants can benefit different farming systems, from small-scale rural farmers to intensive farms, allowing them to explore more sustainable alternatives to chemicals and antibiotics for aquaculture commodities. As A. theiformis is a medicinal plant traditionally used on Reunion Island and included in the French pharmacopoeia, we suggest that exploring national or traditional pharmacopoeias (Caruso et al. 2013, 2017) can be a rich source of information for pre-screening plant diversity for an eco-friendly aquatic pharmacopoeia.
Acknowledgement
Authors would awknoledge Prof. Angela Lusiastuti from Research Center for Veterinary Science, National Research and Innovation Agency (Indonesia) to provide us the bacterial strain used in this study. This study is a part of project: “Reproduction and pathogen control by phyto-therapy in tilapia cultures located in the southwest of Indian Ocean” (PHYTO-TIL- OI) funded by FEAMP-EU. This is a publication ISEM 2023-220