Introduction
Freshwater lobsters, particularly Cherax quadricarinatus, hold significant potential for aquaculture development in Indonesia. The country’s favorable climate, geography, and advancements in cultivation techniques contribute to its suitability for freshwater lobster farming (Jones, Le Anh, and Priyambodo 2019). The increasing consumer demand for freshwater lobsters as a food source and ornamental aquarium species presents attractive opportunities for processing businesses (Lukito and Prayugo 2007). Freshwater lobsters have gained popularity in recent years, both for consumption and as decorative species in aquariums. The market price for consumption-sized lobsters ranges from Rp. 200,000 to Rp. 300,000 per kilogram (Bachtiar 2006). Domestic consumption of freshwater lobsters in 2005 reached an estimated 6-8 tons per month, with Jakarta being the main consumer, absorbing 2-3 tons per month. International demand for Indonesian freshwater lobsters comes from countries such as Japan, Hong Kong, Malaysia, Singapore, the United States, Germany, and several European nations.
While freshwater lobsters are known for their disease resistance, they are still susceptible to bacterial infections. One prevalent disease is Motile Aeromonas Septicemia (MAS), caused by the bacterium Aeromonas hydrophila. MAS symptoms include passive behavior, limb and claw detachment, small wounds on the body, hemorrhaging, ulcers, fluid accumulation, anemia, and internal organ damage (Lukito and Prayugo 2007). Traditional methods to combat MAS, such as antibiotic usage, have limitations due to antibiotic resistance and the presence of antimicrobial residues in lobsters (Su et al. 2017). Vaccination is another approach, but its cost and species-specific nature make it impractical for shrimp. Therefore, the use of immunostimulants, substances that can prevent diseases and enhance the immune system’s response to pathogens, becomes necessary. Immunostimulants provide a safe solution for protecting against diseases by boosting the natural (innate) and adaptive immune systems in fish. (Vallejos-Vidal et al. 2016; Vijayaram et al. 2022).
One highly promising immunostimulant is the unmethylated cytidine phosphate guanosine (CpG) motif, specifically CpG oligodeoxynucleotides (CpG-ODNs). CpG-ODNs mimic bacterial CpG motifs and have demonstrated immune-enhancing properties in mammals, fish, and shrimp (Chen, Xiang, and Shao 2007; Krieg 2002; Tassakka and Sakai 2004). CpG-ODNs activate the innate immune response by binding to Toll-like Receptor 9 (TLR9) on cell membranes, triggering signaling pathways and cytokine production (Sun et al. 2014). While research on the role of CpG-ODNs in the immune systems of aquatic organisms is limited, studies have shown their potential in enhancing immune responses and disease resistance in fish. CpG-ODNs increase the expression of immune genes in fish lymphoid organs and enhance vaccine effectiveness (Kanellos, Sylvester, et al. 1999; Tassakka et al. 2006; Tassakka and Sakai 2004). However, research specific to economically important freshwater crustaceans like Cherax quadricarinatus is lacking.
This research aims to investigate the potential of CpG-ODNs (CpG-ODN 1668, 2133, and 2006) in enhancing the immune response of C. quadricarinatus and identify specific CpG-ODN sequences that exhibit the strongest immunostimulatory effects. Furthermore, the study seeks to evaluate the protective abilities of the most effective CpG-ODN against A. hydrophila infection. By conducting this research, we aim to contribute to scientific knowledge and technological advancements in the field of freshwater lobster farming.
Material and Method
Freshwater lobsters and CpG ODNs
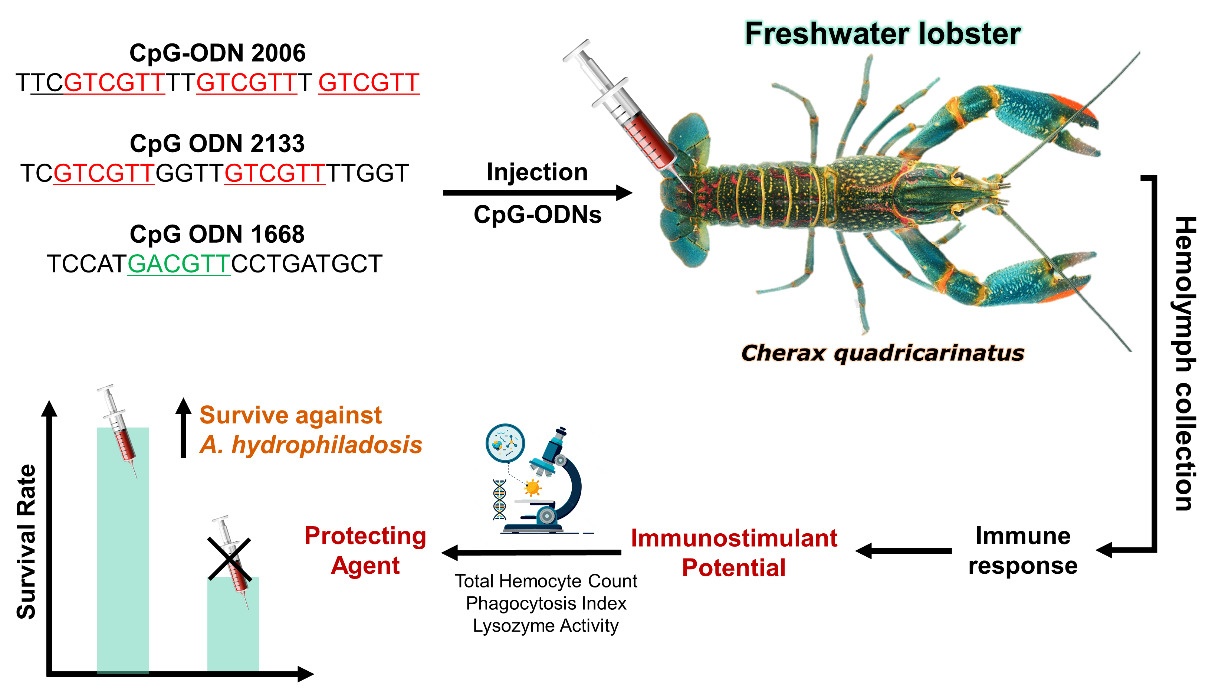
A total of 260 freshwater lobsters (C. quadricarinatus) were collected from a freshwater hatchery in Politani Pangkep, South Sulawesi, Indonesia, with an average weight of 6.38 ± 0.69 g (Figure 1). Lobsters underwent a two-week acclimation period in 1-ton fiber tanks with twice-daily feedings to ensure adaptation before proceeding to immunostimulant testing. Three types of CpG-ODN were used in the study: CpG-ODN 2133 (TCGTCGTTGGTTGTCGTTTTGGT), CpG-ODN 1668 (TCCATGACGTTCCTGATGCT), and CpG-ODN 2006 (TTCGTCGTTTTGTCGTTT GTCGTT) (PT. Genetika Science Indonesia). The CpG ODNs were prepared in accordance with the manufacturer’s guidelines. Each CpG-ODN obtained from the manufacturer was diluted to achieve a concentration of 50 μg/mL, which was subsequently used for injections in each treatment performed on the lobsters.
LD50 Test of A. hydrophila Bacteria
The LD50 test of A. hydrophila bacteria was conducted by serial dilution of a 24-hour-old bacterial culture in phosphate-buffered saline (PBS) solution. Lobsters were infected with a 0.1 mL injection of the diluted bacterial solution. The LD50, causing 50% mortality, was determined to be a bacterial density of 107.
Stage 1: Evaluation of CpG-ODN Potential in Enhancing Immune Response
We adopted a Randomized Complete Design (RCD) involving four distinct treatments (CpG-ODN 2133, CpG-ODN 1668, CpG-ODN 2006, and a control group). Within each treatment, a group of three individual lobsters received a single injection of the respective CpG-ODN, forming a singular experimental unit. This systematic procedure was replicated in triplicate, encompassing key stages such as haemolymph collection, phagocytosis assay, and lysozyme activity assessment. Lobsters were injected with CpG-ODN solutions (50 µg/mL) or a PBS control (Figure 2). The immune responses, including Total Hemocyte Count (THC), Phagocytosis Index (PI), and Lysozyme activity, were assessed before injection and at 1, 3, 5, and 7 days post-injection. Hemolymph collection involved withdrawing 0.1 mL of hemolymph from the base of the first swimmeret using a 1 mL syringe filled with 3.8% Na-citrate anticoagulant. THC was determined using a hemocytometer, while the Phagocytosis Index and Lysozyme activity were assessed through specific assays.
Hemolymph Collection
Hemolymph was collected by withdrawing 0.1 mL from the base of the first swimmeret using a 1 mL syringe containing 0.3 mL of 3.8% Na-citrate anticoagulant (Figure 3). The hemolymph was then homogenized by gently shaking. The first drop was discarded, and subsequent drops were placed on a hemocytometer. The total number of cells per mL was counted under a light microscope at 400x magnification. The produce was examined as described by (Jussila et al. 2001).
Measurement of Total Hemocyte Count (THC)
For THC determination, the collected hemolymph was homogenized and subsequent drops were placed on a hemocytometer. The total cell count per mL was then counted using a binocular microscope at 400x magnification (Anderson, Siwicki, et al., 1995).
Phagocytosis Assay
To calculate the Phagocytosis Index, 0.1 mL of lobster hemolymph was mixed with 25 µL of 24-hour-old Staphylococcus sp. bacteria in a microplate and incubated for 20 minutes. Then, 5 µL of the mixture was smeared onto a glass slide, fixed with 100% methanol for 5 minutes, and stained with 10% Giemsa for 15 minutes. The phagocytic activity was examined as described by (Yoshida et al. 1993). Following rinsing and drying, the percentage of phagocytic cells displaying phagocytosis was determined under a microscope (Anderson, Siwicki, et al., 1995). The Phagocytosis Index was calculated using the formula:
Phagocytic Index=Number of phagocytic cellsTotal number of observed cells×100
Lysozyme Activity
To measure Lysozyme activity, Micrococcus luteus bacteria were cultured in 3 mL of Tryptate Soy Broth (TSB) for 24 hours. Then, 1940 µL of the M. luteus solution was added to 60 µL of PBS with pH 7.4 as the positive control. Similarly, 1940 µL of the M. luteus solution was mixed with 60 µL of lobster hemolymph for each treatment group. A negative control consisting of 200 µL of dissolved water was also prepared. After vortexing for approximately 20 seconds, each sample (200 µL) was transferred to a well in a microplate and spectrophotometrically measured at a wavelength of 450 nm. The plate was then incubated at 30°C for 15 minutes. The lysozyme activity was assayed by the turbidimetric method described by (Parry, Chandan, and Shahani 1965). Subsequent measurements were taken at a wavelength of 490 nm to compare the absorbance results with the initial spectrophotometric measurements (Stolen, Fletcher, et al. 1990).
Stage 2: Evaluation of CpG-ODN Potential as Protecting Agents against A. hydrophila
The second phase utilized a Randomized Complete Design (RCD) with three treatments (selected based on Stage 1 results). Within each treatment, a group of three individual lobsters received a single injection of the respective CpG-ODN, forming a singular experimental unit. Lobsters received injections of CpG-ODN solutions (2006 and 2133 motifs) or a PBS control (0.1 mL) into the ventral sinus of the second abdominal segment. A challenge test was performed on the third day by injecting 0.1 mL of A. hydrophila bacteria into the abdominal region. The survival rate of lobsters was recorded daily until the 7th day after the challenge. The challenge test was examined as described by (Watanuki et al. 2006).
The parameter observed in this phase was the survival rate of the test animals. Observations were conducted daily, starting from the day of the bacterial challenge and continued until the 7th day after the challenge. The data on survival rates were obtained by counting the number of lobsters that survived at the end of the study. The survival rate (SR) was calculated using the formula:
Survival Rate (SR)=Number of lobsters at the end of the experimentNumber of lobsters at the beginning of the experiment×100
Data Analysis
The effects of CpG-ODN administration on immunological parameters were analyzed using analysis of variance (ANOVA), followed by post-hoc Tukey’s test for comparisons between treatments.
Results
Investigating the Potential of CpG-ODN as an Immunostimulant
Total Hemocytes Response to CpG-ODN Treatment
The average total hemocytes of the experimental animals is presented here. Statistical analysis of all treatments at all observation times revealed the differential potential of the three CpG-ODNs in enhancing total hemocytes in freshwater lobsters. Figure 4 illustrates that CpG-ODN 2006 exhibited the highest potential in increasing total hemocytes compared to the other CpG-ODNs.
Total hemocytes in the CpG-ODN 2006 treatment increased from day 1 to day 7, with the maximum peak occurring on day 5, reaching a value of 503,333 cells mL-1. CpG-ODN 2133 showed a gradual increase from day 1 and peaked on day 3 with a total hemocytes count of 426,667 cells mL-1, followed by a decrease until day 7. On the other hand, CpG-ODN 1668 only exhibited a weak potential in increasing total hemocytes, reaching a peak of 340,000 cells mL-1 on day 3, while the PBS solution (control) did not induce any increase.
The overall graph of total hemocytes for all treatments on day 7 showed a decrease. The analysis of variance on day 1 indicated a significant effect of CpG-ODN types on total hemocytes (p<0.05), and the Tukey’s post-hoc test showed a significant difference between CpG-ODN 2006 and the other DNA types, while CpG-ODN 2133 and 1668 did not differ significantly from the control. This suggests that CpG-ODN 2006 on day 1 was superior in enhancing total hemocytes in freshwater lobsters compared to the other CpG-ODNs. The analysis of variance on day 3 demonstrated a significant effect of CpG-ODNs on total hemocytes. The Tukey’s test indicated a significant difference between CpG-ODN 2006 and 2133 compared to CpG-ODN 1668 and the control, illustrating that CpG-ODN 2006 and 2133 had good abilities in enhancing total hemocytes on day 3. On day 5, the analysis of variance revealed a significant effect of CpG types on total hemocytes (p<0.05). The Tukey’s test showed that CpG-ODN 2006 differed significantly from the other CpG-ODNs, indicating that CpG-ODN 2006 had a greater impact in increasing total hemocytes compared to the other CpG-ODNs. On day 7, the analysis of variance showed that CpG types did not have a significant effect on total hemocytes (p>0.05), which can be attributed to the declining total hemocytes count in all treatments. These findings suggest that CpG-ODN 2006 and 2133 have the potential to effectively stimulate total hemocytes in freshwater lobsters, particularly during the early stages of treatment.
Phagocytosis Index
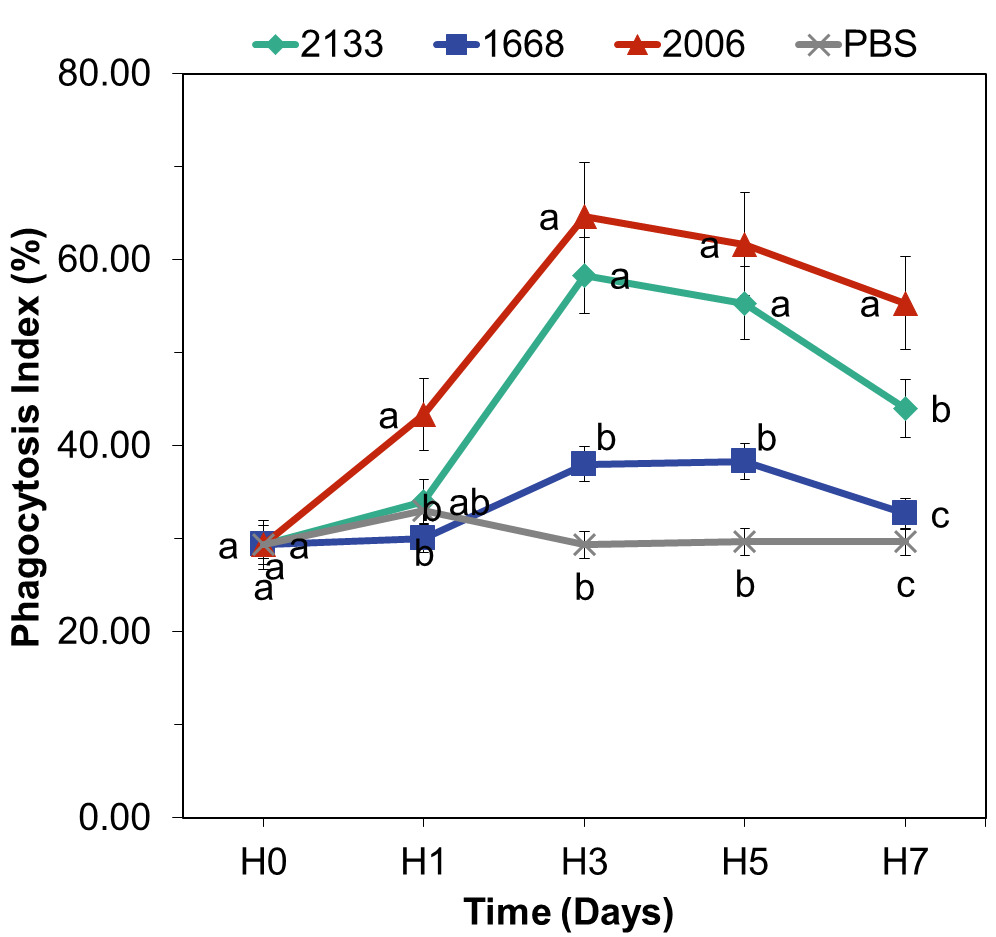
The three types of CpG, namely CpG-ODN 2133, 1668, and 2006, injected into freshwater lobsters had a significant effect on increasing the phagocytosis index. The graph depicting the increase in the phagocytosis index over each observation day can be seen in Figure 5.
Among them, CpG-ODN 2006 showed the highest potential in enhancing the phagocytosis index, with a value of 64.67%, followed by CpG-ODN 2133 with a value of 58.33%. The phagocytosis index reached its maximum point on day 3 and subsequently decreased from day 5 to day 7. On the other hand, CpG-ODN 1668 exhibited a low potential in enhancing the phagocytosis index compared to CpG-ODN 2006 and 2133. Statistical analysis using ANOVA revealed a significant influence of CpG-ODN on the phagocytosis index on days 1, 3, and 5. Tukey’s post-hoc test further confirmed significant differences between CpG-ODN 2006 and 2133 compared to CpG-ODN 1668 and the control group. On day 7, the ANOVA results showed a significant effect of CpG DNA types on the phagocytosis index. Further analysis indicated that CpG-ODN 2006 differed significantly from CpG-ODN 2133, CpG-ODN 1668, and the control group. Statistical tests conducted over the entire observation period demonstrated that CpG-ODN 2006 exhibited specific sequences in enhancing the phagocytosis index. The phagocytosis index values across the observation period showed a quadratic trend (p<0.05), with the turning point (highest response value) occurring on day 3, followed by a gradual decline.
Lysozyme Activity
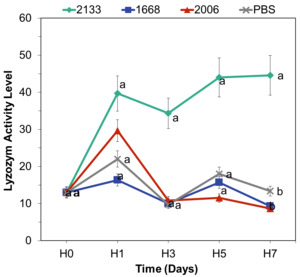
Before the administration of CpG-ODN, the average lysozyme activity was 13 units. On the first day of observation, there was an increase in lysozyme activity, with the highest value observed in the CpG-ODN 2133 treatment (40 units), followed by CpG-ODN 2006 (30 units), CpG-ODN 1668 (13 units), and PBS (13 units). The lysozyme activity graph depicted a continuous increase from day 1 to day 7 (Figure 6).
The analysis of variance (ANOVA) results for all treatments revealed no significant effect of CpG-ODN on the increase in lysozyme activity from day 1 to day 5. However, on day 7, the ANOVA results showed a significant effect of CpG-ODN types on total lysozyme activity (p<0.05). Post-hoc Tukey’s test indicated that CpG-ODN 2133 had the highest lysozyme activity and significantly differed from the other CpG-ODN types. This suggests that CpG-ODN 2133 has the greatest potential for enhancing lysozyme activity in C. quadricarinatus.
Investigating the Potential of CpG-ODN as a Protective Agent
The survival rates of the test shrimp at the end of the study consistently showed that the CpG-ODN 2133 treatment had the highest value of 98.33%, followed by CpG-ODN 2006 with a value of 96.67%, and PBS with 45.00%. The survival rate graph for all treatments is presented in Figure 7.
The analysis of variance (ANOVA) results showed that the administration of CpG-ODN had a significant effect on the survival rate of lobsters. The post-hoc Tukey’s test indicated that CpG-ODN 2133 and 2006 had significant differences compared to PBS (control). Both CpG-ODNs demonstrated the ability to act as protecting agents against the pathogenic bacterium A. hydrophila, as evidenced by the high survival rates of C. quadricarinatus. However, CpG-ODN 2133 exhibited a higher potential compared to CpG-ODN 2006.
Discussion
Freshwater lobsters, specifically C. quadricarinatus, were chosen as the experimental model in this study due to the prevalence of MAS disease caused by the bacterial infection A. hydrophila. MAS disease poses a significant threat to freshwater lobster populations, leading to severe economic losses in aquaculture. Hence, there is a critical need to explore potential strategies for enhancing the immune response and developing protective agents against this bacterial infection. The primary objective of this research was to evaluate the potential of CpG-ODNs as immunostimulants and protective agents in Cherax quadricarinatus lobsters. To accomplish this, the lobsters were divided into different treatment groups and injected with three types of CpG-ODN (2133, 2006, and 1668), while a control group received injections of phosphate-buffered saline (PBS). By conducting measurements of Total Hemocyte Count (THC), performing a Phagocytosis Assay, and assessing Lysozyme Activity, we aimed to gain insights into the immunostimulatory effects of CpG-ODNs and identify specific CpG-ODN sequences that enhance the immune response in freshwater lobsters. Furthermore, we sought to evaluate the potential of the CpG-ODN that elicited the highest immune response as a protective agent against A. hydrophila infection.
In the context of crustacean aquaculture, it is crucial to delve deeper into the implications of the assessed parameters. Total hemocyte count (THC) emerges as a pivotal indicator of immune response and overall health in freshwater lobsters. Variations in THC levels can potentially influence the organism’s resistance to infectious diseases and environmental stressors. Drawing insights from studies on crayfish and other crustaceans, it becomes evident that changes in THC may impact immune competence and overall fitness. This broader perspective underscores the practical significance of our results in the aquaculture context, offering insights for the management of crustacean health and well-being (Purbiantoro et al. 2023; Thayappan et al. 2017). Furthermore, the interconnected roles of phagocytic activity and lysozyme activity are integral to the innate immune defense mechanism of crustaceans. The collaborative functions of these parameters contribute to the effective elimination of microbial threats (Chung and Kocks 2012). Phagocytosis, the internalization of particles or microorganisms into cells, leads to the formation of phagosomes, where degradative enzymes are released to facilitate particle elimination. This process is accompanied by the generation of Reactive Oxygen Intermediates (ROIs), known as the respiratory burst, further enhancing the microbicidal potential (Rosales and Uribe-Querol 2017; Uribe-Querol and Rosales 2017). The synergy between phagocytosis and lysozyme activity presents a multifaceted defense mechanism, reinforcing the organism’s ability to combat infections.
CpG-ODNs, as immunostimulants, protective agents, and vaccine adjuvants in vertebrates, possess specific sequences or optimal motifs that vary from one species to another. For instance, the sequence “TTCGTT” can enhance immune responses in humans, yet it proves ineffective in eliciting immune responses in fish. Interestingly, different CpG-ODN sequences can act as immunostimulants in fish; for example, they can enhance phagocyte activity in species like grass carp, common carp, rainbow trout, and even trigger immune responses in crustaceans (Tassakka and Sakai 2004). CpG-ODNs, being synthetic oligodeoxynucleotides with unmethylated CpG motifs, emulate bacterial DNA patterns. These motifs are recognized by Toll-like Receptor 9 (TLR9), a pivotal element of the innate immune system present in various immune cells, including crustacean hemocytes (Hemmi et al. 2000). The binding of CpG-ODNs to TLR9 initiates signaling cascades that culminate in the upregulation of immune-related genes and the synthesis of pro-inflammatory cytokines, thereby amplifying the immune response (Bode et al. 2011). Based on the described mechanisms observed in mammalian immune systems, interactions between CpG-ODNs and the lobster’s immune system likely involve the recognition of unmethylated CpG motifs by potential Toll-like receptors, such as TLR9. If present in lobsters, TLR9 could be located within endosomes of immune cells. Upon uptake of CpG-ODNs via endocytosis, these molecules could engage TLR9 and initiate a signaling cascade similar to the MyD88-dependent pathway seen in mammals. This activation could lead to the release of pro-inflammatory cytokines and other immune mediators, triggering an enhanced innate immune response (Sun et al. 2014). However, further investigation is necessary to ascertain the presence and functionality of TLRs in lobsters, confirming these interactions and their effects on the lobster’s immune system.
The analysis of total hemocyte count (THC) provided significant insights into the immunostimulatory effects of CpG-ODNs in C. quadricarinatus lobsters (Figure 4). Our experimental design and analysis revealed variations in immune response enhancement among the different CpG-ODN types tested. We observed differences in the increase of total hemocytes across different time points within each treatment group, indicating distinct effects of the treatments on total hemocyte count. Based on the statistical results, CpG-ODN 2006 significantly differed from the other CpG-ODNs and the control in enhancing total hemocytes at each observation time, while CpG-ODN 2133 only differed significantly from the other CpG-ODNs on day 3, and CpG-ODN 1668 did not show significant differences in enhancing total hemocytes. CpG-ODN 2006 has shown to be the most effective in enhancing total hemocytes in freshwater lobsters (Lu et al. 2006). This finding is consistent with previous research, which demonstrated that CpG-ODN 2006 effectively increased proPO in giant freshwater prawns (Macrobrachium rosenbergii) three hours post-injection (Sung, Chen, and Liu 2008; Sung et al. 2009). CpG-ODN 2133 also exhibited an effect in enhancing the immune response, particularly total hemocytes count, in freshwater lobsters (Chen, Xiang, and Shao 2007). In contrast, CpG-ODN 1668 did not show any effect in increasing hemocytes count, consistent with previous research, which demonstrated that CpG-ODN 1668 specifically enhanced responses in common carp (Cyprinus carpio) and grass carp (Ctenopharyngodon idellus) (Meng, Shao, and Xiang 2003; Tassakka et al. 2006; Tassakka and Sakai 2004).
Existing literature suggests that CpG-ODNs have a potent ability to activate the functions of leukocytes. Leukocytes, critical components of the immune system, are particularly essential in organisms like crustaceans that lack erythrocytes. The immune defense mechanisms in crustaceans are less developed compared to those in other vertebrate fish. Specifically, crustaceans lack adaptive memory, meaning they cannot produce immunoglobulins. Consequently, they seem to heavily rely on their innate immune defense system (Roch 1999). The innate immune system, often referred to as the natural or non-specific defense system, plays a pivotal role by orchestrating both cellular and humoral components in a collaborative effort to detect and eliminate potentially harmful foreign organisms within the host’s (Jiravanichpaisal, Lee, and Söderhäll 2006). This defense system is vital for maintaining the well-being of organisms, including crustaceans, in their natural habitats.
The cellular part of the immune system acts as the first line of defense. Hemocytes, which include hyalocytes, granulocytes, and semi-granulocytes, play a central role in executing different immune responses such as gobbling up invaders, forming protective capsules around them, and creating nodules. These actions directly help eliminate harmful microbes and particles from the host’s body. Moreover, hemocytes do more than just defend – they also have various other roles like healing wounds, aiding in blood clotting, strengthening the outer shell, and managing the body’s energy and protein needs. The humoral part of the innate immune system is just as important. Molecules like anticoagulants, agglutinins, phenoloxidase enzymes, antimicrobial peptides, and protease inhibitors are stored in hemocytes (Kankamol and Salaenoi 2018). These molecules get activated and released when needed to help fight infections and assist in healing wounds, thus promoting the overall health of crustaceans
In this context, the significance of total hemocyte count (THC) becomes apparent as an important gauge of an organism’s immune response and general health, as it enhances the formation of phagocytic cells and initiates the flow of proPO, which plays a crucial role in controlling microbial attacks within the body (Liu et al. 2020; Mahasri et al. 2018). Increased hemocytes in the body signifies an improved immune resilience in freshwater lobsters, while their vulnerability to infectious diseases decreases. Total hemocytes is believed to influence the host’s response against foreign substances and various responses to infections, environmental changes, and ecdysis in most crustaceans (Kongnum and Hongpattarakere 2012). Hemocytes comprises hyalin, granular, and semi-granular cells and exhibits phagocytic activity while storing the prophenoloxidase (ProPO) enzyme. These cells can be stimulated to induce exocytosis and enzyme release. Their functions include encapsulation, initiation of proPO flow, and phagocytosis. Hemocytes possesses inhibitory enzymes necessary for regulating proteolytic flow and produces cytotoxic molecules such as lysozyme, esterase, phospholipase, and peroxidase (Zhang, Shao, et al., 2006).
Furthermore, the phagocytosis assay results provided additional evidence of the immunostimulatory effects of CpG-ODNs (Figure 5). CpG-ODN 2006 and CpG-ODN 2133 exhibited notable capabilities in enhancing the phagocytosis index, indicating an improved ability of hemocytes to engulf and eliminate foreign particles. The statistical analysis conducted from day 1 to day 7 led to the conclusion that CpG-ODN 2006 is the most effective CpG-ODN in enhancing the immune response of C. quadricarinatus (Lu et al. 2006). This finding aligns with the study by Sung et al. (2009) which reported that a dose of 5 µg CpG-ODN 2006 effectively increased the proPO (prophenoloxidase) levels in M. rosenbergii three hours post-injection. These two studies reinforce the specificity of CpG-ODN 2006 in enhancing the phagocytosis index in C. quadricarinatus, considering that the increase in the phagocytosis index is essentially an implication of increased hemocytes levels. In crustaceans, hemocyte cells possess biological properties and functions similar to those of macrophages, granulocytes, and vertebrate natural killer cells (Wałajtys-Rode and Dzik 2017). These cells play roles in phagocytosis, encapsulation, nodule formation, wound healing, coagulation, and proPO activation. Additionally, they aid in the production of adhesive molecules, agglutinins, and antimicrobial peptides (AMP) (Bachère, Destoumieux, and Bulet 2000; Tassanakajon et al. 2013). CpG-ODN 2006 not only acts as a specific immunostimulant in crustaceans but also exhibits immunostimulatory effects in fish. Strandskog, Ellingsen, and Jørgensen (2007) demonstrated that CpG-ODN 2006 effectively enhances the activities of interferon α/β and cell proliferation in Atlantic salmon.
CpG-ODN 2133 significantly increased the phagocytosis index compared to the control group from day 1 to day 5, indicating its effectiveness as an immunostimulant in lobsters, although its potential is lower than that of CpG-ODN 2006. Studies conducted on rainbow trout injected with CpG-ODN 2133 at a dose of 0.4 µg demonstrated an effective increase in the phagocytosis index on the second day post-injection (Carrington and Secombes 2007). Another study reported that CpG-ODN 2133, which is one of the components of plasmid DNAs, effectively enhances the immune response in Crucian carp (Carassius auratus) (Chen, Xiang, and Shao 2007). These findings support the significant effect of CpG-ODN 2133 at a dose of 0.5 µg on the phagocytosis index in C. quadricarinatus, albeit with a lower capability compared to CpG-ODN 2006.
Phagocytosis involves the internalization of foreign materials and serves as the primary cellular defense mechanism in invertebrates. It is performed by semi-granulocytes and granulocytes, which consist of various stages, including chemotaxis, attachment, engulfment, pathogen destruction, and exocytosis (Kondo et al. 1998). An example of phagocytic hemocyte cells can be found in Figure 8.
The observed decrease in the phagocytosis index on the seventh day is attributed to the phagocytic activity. Phagocytic cells are partially destroyed along with bacteria after undergoing various phagocytosis processes. According to Melcarne (2019), the phagocytosis process involves the following stages such as particle attachment to cell surfaces, engulfment and bacterial destruction and digestion. Degranulation can be followed by cell lysis. As a result, some hemocytes cells may be lost during the degranulation process, leaving their degraded contents within the fibrous extracellular matrix (Aprelev et al. 2019; Dushay 2009). Phagocytic cells that have not been destroyed together with pathogens during the phagocytosis process subsequently leave the hemolymph. Braak & de (2002) stated that hemocytes phagocytic cells can leave circulation after performing phagocytosis and enter the heart, supportive tissues, gills, and other haemal sinuses. Immuno-staining studies have shown that many degranulated hemocytes cells are present in lymphoid organs, resulting in a fibrous layer forming on the outside of tubule walls. This condition can contribute to a reduced concentration of hemocytes in the hemolymph of diseased animals or after infection with foreign substances.
The evaluation of lysozyme activity provided further evidence of the immunostimulatory effects of CpG-ODNs (Figure 6). Among the tested CpG-ODN types, CpG-ODN 2133 demonstrated the highest lysozyme activity, significantly surpassing the activity levels of the other CpG-ODN types. This finding aligns with previous research conducted on rainbow trout, where the administration of CpG-ODN 2133 also resulted in a significant increase in lysozyme activity on days 7 and 10 after injection (Carrington and Secombes 2007). The significant difference in CpG-ODN 2133 activity on day 7 is related to cell maturation processes. Cells reach their peak maturation on the seventh day, and it is at this point that lysozyme enzymes begin to function actively as part of the non-specific immune defense mechanism. The notable effect of CpG-ODN 2133 on lysozyme activity in C. quadricarinatus is consistent with Chen’s findings which demonstrated an increase in lysozyme activity following the administration of recombinant plasmids containing CpG-ODN 2133 in giant freshwater prawns (Chen, Xiang, and Shao 2007). CpG-ODN 1668 did not have any effect on lysozyme activity, likely because this CpG-ODN is specific to fish (Strandskog, Ellingsen, and Jørgensen 2007; Tassakka et al. 2006; Tassakka and Sakai 2004).
Lysozyme serves as an indicator to assess the level of immunity or immune response in shrimp given immunostimulants. It is found in the serum and mucus of fish, particularly in tissues rich in leukocytes such as the kidney, stomach, and spleen. Monocytes, macrophages, and neutrophils are the main sources of lysozyme (Ayyappan et al. 2020; Wenzel et al. 2011). In shrimp, lysozyme can originate from hyaline cells, and it is known that shrimp hemocytes are divided into hyaline (agranular), semigranular, and granular types (Ratcliffe and Rowley 1981). Lysozyme is a non-specific immune response enzyme that plays a vital role in shrimp’s defense mechanism. It cleaves the β-1,4-glycosidic bond between N-acetylglucosamine and N-acetylmuramic acid in peptidoglycan, thereby damaging bacterial cell walls. This process leads to the entry of water into the cell, causing it to swell and eventually burst, a phenomenon known as lysis (Chipman and Sharon 1969). Lysozyme degrades mucopolysaccharides in the Gram-negative bacterial cell wall and modifies the molecular conformation of cell surfaces, enabling their recognition by phagocytic cells. Lysozymes participate in the degradation of microorganisms inside and outside hemocytes, and some also play a role as sterases and chitinases (De-la-Re-Vega, García-Orozco, et al., 2004).
Moreover, our study aimed to evaluate the potential of the CpG-ODN that elicited the highest immune response in C. quadricarinatus as a protective agent against A. hydrophila infection (Figure 7). The ability of CpG-ODN 2133 as a protecting agent against A. hydrophila is closely related to the success of both CpG-ODNs in stimulating the lobster’s immune response, including total hemocyte count, phagocytosis index, and lysozyme activity, all of which contribute to the high survival rate of the test animals. Hemocytes are the primary defense mechanism in shrimps, and an increase in total hemocyte count leads to an enhancement of the humoral and cellular immune defense systems. The role of CpG-ODN 2133 as a protecting agent against A. hydrophila is supported by the research conducted by Carrington (2004) which revealed that CpG-ODN 2133 can enhance kidney leukocyte proliferation and blood leukocyte proliferation in rainbow trout (Oncorhynchus mykiss). Carrington and Secombes (2007) further investigated and concluded that CpG-ODN 2133 acts as a protecting agent against bacteria when tested in vivo on rainbow trout (Oncorhynchus mykiss). The success of CpG-ODN 2133 as a measurable protecting agent, demonstrated by the high survival rates, is essentially an implication of an enhanced immune response. As a result, the entry of pathogens such as A. hydrophila can be effectively controlled by freshwater lobsters.
Conclusions
This study examined the immunomodulatory effects of CpG-ODN administration on C. quadricarinatus freshwater lobsters. CpG-ODN 2006, characterized by the TTCGTCGTTTTGTCGTTTGTCGTT motif, significantly increased total hemocyte count and enhanced the phagocytic index, making it the most effective stimulant. CpG-ODN 2133, with the TCGTCGTTGGTTGTCGTTTTGGT motif, displayed notable activity in enhancing lysozyme function. Both CpG-ODN 2006 and 2133 showed potential as defense agents against Aeromonas hydrophiladosis. These findings emphasize the species-specific immunostimulatory role of CpG-ODN 2006, specifically targeting freshwater lobsters. This study underscores the importance of CpG-ODN as a promising immunostimulant in aquaculture. Future investigations should explore additional immune parameters, such as respiratory burst and immune gene expression, to gain a comprehensive understanding of CpG-ODN’s underlying immunomodulatory mechanisms. Moreover, exploring different types of CpG-ODN on various aquatic species would enhance our knowledge of species-specific immunomodulatory properties.
Acknowledgements
We are grateful for the close collaboration between Universitas Hasanuddin and Pangkep State Polytechnic of Agriculture in completing this study. All experimental procedures involving freshwater lobsters were conducted in accordance with ethical guidelines and regulations established by Indonesian government

_used_in_the_study.jpeg)





_chemotaxis_process_(2)_adhesion_p.png)
_used_in_the_study.jpeg)





_chemotaxis_process_(2)_adhesion_p.png)