1. Introduction
The marbled flounder (Pseudopleuronectes yokohamae) is widely distributed in China, Japan, and North Korea (C. Zhang et al. 2016) and serves as a key aquaculture species in northern China, especially in Shandong and Dalian (Cui et al. 2019). Due to its favorable traits—such as low-temperature tolerance, strong adaptability, and high nutritional value—it has been artificially bred since the 1960s (Cho et al. 2021). However, recent declines caused by disease, overfishing, and environmental stressors have led to significant economic losses (Z. Zhang et al. 2022), highlighting the need to investigate disease control strategies.
Skin ulceration syndrome (SUS) is a major disease threat in aquaculture, particularly under high-density farming conditions where physical injuries increase susceptibility (Yang et al. 2023). Similar symptoms have been reported in other flatfish and species such as Cynoglossus semilaevis (Zuo et al. 2023), Epinephelinae, and Pseudopleuronectes americanus (Bodammer 2000), with ulcers often leading to high mortality and reduced market value (Xu et al. 2015). Treatment approaches vary, with antibiotic immersion being commonly used.
Among the pathogens, Vibrio spp. are widespread marine bacteria with many known fish and shellfish pathogens, including V. harveyi, V. parahaemolyticus, V. alginolyticus, V. vulnificus, and V. splendidus (Lin et al. 2010; Ghasemieshkaftaki et al. 2023). Some, such as V. parahaemolyticus, cause AHPND in shrimp (Fadel et al. 2025), while others infect a range of hosts including Oreochromis niloticus (Elgendy et al. 2022) and Scorpaena porcus (Eissa et al. 2015). V. splendidus, in particular, is highly genotypically diverse and has been implicated in disease outbreaks in turbot (Thomson et al. 2005), cod (Gadus morhua) (Reid et al. 2009), and bivalves (Dubert, Barja, and Romalde 2017), as well as ectoparasitic hosts (Jensen et al. 2003; Gulla et al. 2015). It is also linked to SUS outbreaks in sea cucumbers (C. Li et al. 2012), underscoring its broad host range and economic impact.
Due to the host- and environment-specific characteristics of V. splendidus, further study of its pathogenic traits is crucial for disease control. In this study, we isolated and identified the causative agent of SUS in P. yokohamae using morphological analysis, biochemical profiling, 16S rRNA sequencing, antibiotic susceptibility testing, and bacterial growth experiments (Liao et al. 2024), aiming to provide a scientific basis for the prevention and treatment of SUS in cultured marbled flounder.
2. Materials and Methods
2.1. Animals
Diseased P. yokohamae were collected from Xinchangxing Market in Shahekou District, Dalian city, Liaoning Province, China, and used for the isolation and detection of pathogenic bacteria. Prior to bacterial isolation, all diseased individuals underwent microscopic examination and PCR detection for common parasitic infections and bacterial pathogens to rule out co-infections. Only fish confirmed to be free of co-infections were included in the study. Healthy P. yokohamae specimens, exhibiting intact body surfaces, normal pigmentation, and good vitality, with an average weight of 150±20 g, were selected for the artificial infection experiment. Before the experiment, the P. yokohamae were acclimated at the Key Laboratory of Northern Mariculture of the Ministry of Agriculture at Dalian Ocean University. The breeding tanks were filled with naturally filtered seawater obtained through sedimentation and sand filtration. The water temperature was maintained at 15 ± 1°C, the salinity was 30, and the pH was 7.4 ± 0.1. During the temporary breeding period, sandworms were fed twice a day, each time at 3±1% of body weight to ensure their adaptation to the new environment. Feeding was stopped 12 hours before the artificial recall experiment.
2.2. Pathogenic and histopathological properties
Clinical and postmortem examinations of naturally infected Pseudopleuronectes yokohamae were conducted to identify gross lesions and internal abnormalities, following the standard procedures described by Jiang (X. Jiang et al. 2022). To exclude co-infections, all diseased fish underwent microscopic and PCR screening for common pathogens. Nine infected and three healthy laboratory-acclimated fish were selected. After rinsing with sterile saline, fish were dissected aseptically, and gill, liver, and intestinal tissues were fixed in Davidson’s solution. Samples were processed using standard histological techniques, stained with H&E, and examined under a light microscope (Olympus, Japan). Images were acquired with Leica LAS X 3.1.1 software.
2.3. Phenotypic observation of pathogenic bacterial isolates from fish specimens
Bacteria were isolated from the skin ulcers, liver, and spleen of diseased fish using Tryptic Soy Broth (TSB; Hopebio, China), and then streaked onto 2216E agar (Hopebio, China) for incubation at 16 °C for 12–16 h. Single colonies were subcultured on 2216E agar at least three times to obtain pure strains, which were subsequently transferred to thiosulfate-citrate-bile salt-sucrose (TCBS) agar for colony morphology observation. Identification of Vibrio species followed Bergey’s Manual (9th Edition) (Mahbub, Paul, and Ahmed 2011). Gram staining was performed during the late logarithmic phase according to Cruickshank (Cruickshank et al. 1975), and bacterial morphology was observed under a microscope to differentiate Gram-positive (purple) and Gram-negative (red) cells.
2.4. Determination of biochemical characteristics
Purely cultured colonies were selected to prepare a bacterial suspension under sterile conditions, and ID 20E (BioMérieux, France) Enterobacteriaceae bacteria identification test strips were utilized to identify the biochemical characteristics of bacteria (Varettas, Mukerjee, and Schmidt 1995) following the manufacturer’s protocol. In brief, 100 μL of bacterial suspension was added to the biochemical reaction cup, and mineral oil was added to create an anaerobic environment. Afterward, the test strips were covered. After incubation at 36°C for 18–24 h, the identification reagent was added dropwise according to the manufacturer’s instructions. To identify Vibrio species, a series of biochemical tests were performed via API 20E strips, each containing 20 microtubes for specific tests. These tests collectively provided valuable information on the metabolic characteristics of the bacterial isolate, aiding in its identification (Aly et al. 2023).
2.5. Molecular Strategies for Bacterial Characterization
Genomic DNA was extracted from bacterial cultures using the Bacterial Genomic DNA Extraction Kit (TIANGEN, China), following the manufacturer’s protocol. DNA concentration and quality were assessed via agarose gel electrophoresis and NanoDrop spectrophotometry. The 16S rRNA gene was amplified using universal primers 27F and 1492R (H. Jiang et al. 2006) on a ProFlex™ PCR System (Thermo Fisher, USA). To improve species-level resolution, the gyrB gene was also amplified using primers gyrBF and gyrBR (Yamamoto and Harayama 1995; Wang et al. 2007). Primer sequences and PCR conditions are listed in Table 1. PCR products were separated by 1% agarose gel electrophoresis using a DL2000 DNA Marker (Takara, Japan), visualized under UV light, and purified for sequencing (Sangon Biotech, Shanghai, China). Consensus sequences were aligned with NCBI database references, and phylogenetic trees were constructed using the neighbor-joining method based on 16S rRNA and gyrB gene sequences.
2.6. Antimicrobial resistance analyses
Antimicrobial susceptibility was evaluated using the Kirby–Bauer disk diffusion method, following the Clinical and Laboratory Standards Institute (CLSI) M100 guidelines (CLSI 2020). Marine bacterial isolates were cultured on Mueller–Hinton agar (MHA; Hopebio, China) supplemented with 1.5% NaCl. Bacterial suspensions were adjusted to a turbidity equivalent to 0.5 McFarland standard and evenly inoculated onto the surface of MHA plates. Antibiotic discs were applied to the agar surface with sterile forceps after drying for 3–5 minutes. The antibiotics tested included norfloxacin, ciprofloxacin, enrofloxacin, ceftazidime, tetracycline, doxycycline, oxytetracycline, kanamycin, gentamicin, erythromycin, amoxicillin, sulfadiazine-trimethoprim, and florfenicol. Plates were incubated at 28 °C for 22–24 hours, after which the diameters of the inhibition zones were measured. All assays were performed in duplicate. Results were interpreted according to the CLSI VET03 guidelines (CLSI 2020).
2.7. Bacterial growth experiment
All strains (Isolated strain 1, Isolated strain 2, and Isolated strain 3) were cultured at 15°C overnight. Three hundred microliters of each bacterial culture was subsequently extracted and diluted to a ratio of 1:100. The mixture was sequentially inoculated into 30 mL of fresh 2216E Liquid Medium (Hopebio, China) and then placed in an incubator at 15 C with shaking at 200 rpm. The optical density at 600 nm was determined hourly for 10 h via a spectrophotometer (Xie et al. 2020). Sterile 2216E broth was used as a blank control, and all OD₆₀₀ measurements were performed using the same calibrated spectrophotometer. Data from triplicate cultures were averaged, and the entire experiment was independently repeated three times.
2.8. Artificial infection experiment
The tested strains were cultured in 2216E liquid medium (Hopebio, China) at 28 °C for 12–16 h and resuspended in sterile saline (0.9% NaCl) to prepare bacterial suspensions. Fish were randomly assigned to three experimental and three control groups, each with three replicates (15 fish per 0.5 m³ bucket) (Zuo et al. 2023; Liao et al. 2024). Experimental groups were intraperitoneally injected with 0.2 mL of bacterial suspension (1×10⁷ CFU/mL) (Ghasemieshkaftaki et al. 2023), while control groups received an equal volume of saline, PBS, or heat-killed bacteria. Fish were maintained at 15 ± 1 °C for 14 days, fed as described in section 2.1, with adjusted feeding as needed. Mortality, clinical signs, and pathological features were recorded (Ghasemieshkaftaki et al. 2023). Moribund and dead P. yokohamae were dissected to examine lesions, and tissues and ascitic fluid were collected for pathogen re-identification via 16S rRNA and gyrB gene PCR and sequencing.
2.9. Ethics statement
The present study was carried out following the recommendation of the ARRIVE guidelines for animal research. The Animal Research and Ethics Committee of Dalian Ocean University approved this study. Anatomical experiments were performed under anesthesia using ethyl 3-aminobenzoate mesylate (MS-222. Sigma‒Aldrich. USA) to minimize fish discomfort.
3. Results
3.1. Clinical examination
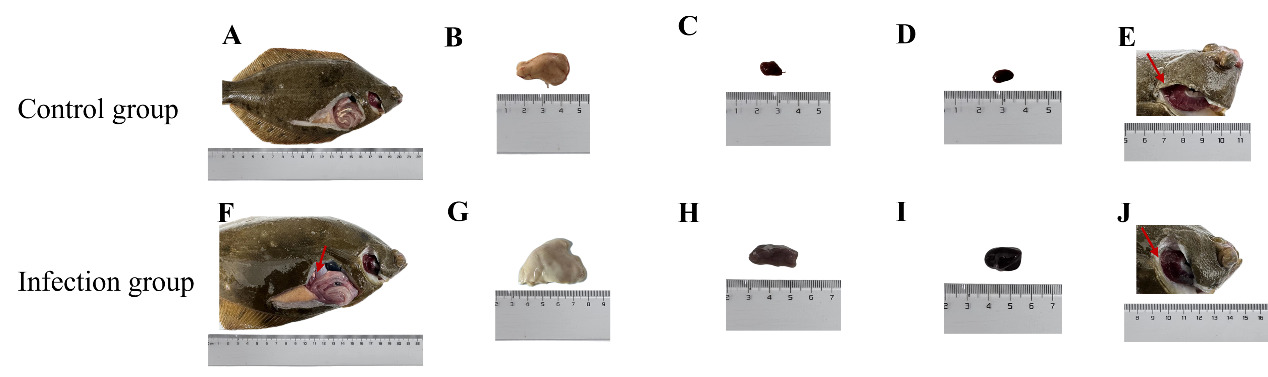
Clinical signs of SUS included anorexia, reduced resistance, abnormal swimming, and lethargy, with ulcer formation and tail bleeding in severe cases (Figure 1). Necropsy revealed pale livers, swollen discolored kidneys and spleens, gill congestion and edema, mucus in gill filaments, thin gut walls, and ascites (Figure 2). Histopathology showed hepatic vacuolation and hyperemia (Figure 3D), gill cell swelling with blood cell infiltration (Figure 3E), and thinning of the mucous layer with increased immune cells and scale loss (Figure 3F).
3.2. Culture conditions and morphological characteristics
The isolates were designated as Isolated strains 1–3. On 2216E medium at 15°C for 24 h, Isolated strain 1 formed circular, orange colonies, while strains 2 and 3 were white. On TCBS agar, strain 1 produced yellow, swarming colonies (2–3 mm), strain 2 formed green colonies (1–2 mm), and strain 3 showed no growth. Microscopically, all isolates were Gram-negative, short, motile rods, either straight or curved (Supplementary Figure 1).
3.3. Biochemical identification
Three bacterial isolates were identified using the API 20E system (bioMérieux, France) based on their biochemical profiles. Isolate 1 tested positive for several sugar fermentations (GLU, MAN, SAC, ARA), LDC, ODC, and IND, with an API code of 6144600, indicating Vibrio spp. Isolate 2 was only positive for citrate (CIT), with an API code of 0200000, suggesting Pseudoalteromonas spp. Isolate 3 showed positive reactions for GLU, LDC, H₂S, and GEL, with an API code of 1006100, identifying it as Shewanella spp. These API codes enable species-level presumptive identification based on metabolic traits (Table 2).
3.4. Phylogenetic analyses
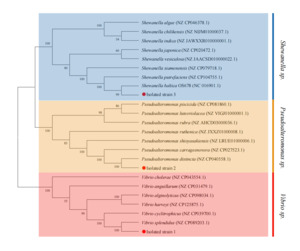
PCR amplification of the 16S rRNA gene from all three isolates yielded ~1,500 bp amplicons. BLAST analysis showed >99% identity with Vibrio splendidus (99.09%), Pseudoalteromonas distincta (99.93%), and Shewanella baltica (99.86%) for Isolates 1–3, respectively. Phylogenetic analysis confirmed clustering with reference strains (Supplementary Figure 2). To improve species resolution, gyrB was also analyzed, revealing 99.49%, 99.31%, and 100% identity with the corresponding species. The phylogenetic tree based on gyrB supported these identifications (Figure 4). Sequences were submitted to GenBank (16S rRNA: PP330039–PP330041; gyrB: PQ189395–PQ189397).
3.5. Antimicrobial resistance analyses
Antimicrobial susceptibility testing of Isolated strains 1, 2, and 3 against 13 antibiotics showed distinct resistance profiles (Table 3). Isolated strain 1 was sensitive to norfloxacin, ciprofloxacin, enrofloxacin, ceftazidime, doxycycline, and florfenicol, but resistant to tetracycline, gentamicin, erythromycin, and amoxicillin. Isolated strain 2 exhibited sensitivity to norfloxacin, ciprofloxacin, enrofloxacin, tetracycline, doxycycline, kanamycin, and gentamicin, but was resistant to ceftazidime, oxytetracycline, erythromycin, amoxicillin, and florfenicol. In contrast, Isolated strain 3 was sensitive to florfenicol, ceftazidime, doxycycline, gentamicin, and trimethoprim, but resistant to ciprofloxacin, ceftazidime, erythromycin, and amoxicillin. The interpretation of antimicrobial resistance was based on the CLSI VET03 guidelines (Supplementary Table 1).
3.6. Bacterial growth patterns of pathogenic bacteria
The strains of Isolated strain 1, 2, and 3 displayed similar representative growth patterns. Nonetheless, the proliferative rate of Isolated strain 1 was significantly greater than that of Isolated strain 2 and Isolated strain 3 (Supplementary Figure 3).
3.7. Artificial recall experiment
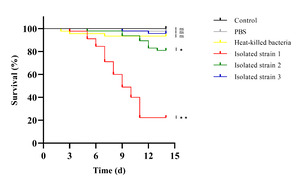
Artificial infection assays showed that only fish injected with Isolated strain 1 developed typical SUS symptoms within 48 hours, while strains 2 and 3 caused only mild behavioral changes. No symptoms appeared in the PBS, heat-killed, or control groups. Kaplan–Meier analysis revealed that strain 1 caused high mortality (~80% by day 12), strain 2 caused moderate mortality (~20%), and the other groups had no significant deaths. Survival in the strain 1 group was significantly lower than all others (p < 0.01), and strain 2 showed a mild but significant effect (p < 0.05) (Figure 5). Strain 1 also matched the dominant bacteria re-isolated from diseased fish, and 16S rRNA and gyrB gene sequencing confirmed it as the causative agent of SUS in P. yokohamae.
4. Discussion
Pseudopleuronectes yokohamae is widely distributed in East Asia, including the Yellow Sea, southern Hokkaido, and Korean coastal regions (Ji, Kim, and Kim 2016), and holds significant commercial value (Huh et al. 2012). Despite its importance, the etiology of skin ulcer syndrome (SUS) remains unclear. In the present outbreak, fish showed clinical and pathological signs such as liver pallor, gill edema, intestinal thinning, and ascites under moderate temperature and salinity conditions. Histopathological lesions included vacuolar degeneration in the liver, epithelial swelling in gills, and immune cell infiltration.
Biochemical and molecular identification revealed that isolates belonged to Vibrio splendidus, Pseudoalteromonas distincta, and Shewanella baltica. The use of gyrB gene sequencing, with higher taxonomic resolution than 16S rRNA (Urdaci et al. 1998; Romalde et al. 1999; Cepeda and Santos 2000; Liu et al. 2021; Kong et al. 2022), clarified species identity.
Among the isolates, V. splendidus exhibited strong pathogenicity. This bacterium is metabolically versatile—capable of fermenting sugars, producing indole (S. Zhang et al. 2017), utilizing L-glutamate (Y. Li, Shi, and Zhang 2023), and secreting metalloproteases like Vsm (Binesse et al. 2008)—all contributing to tissue damage and immune evasion (Mey, Craig, and Payne 2012; Rivera-Posada et al. 2011; J. Li et al. 2020). Its infection patterns are consistent across multiple hosts, including turbot (Gatesoupe, Lambert, and Nicolas 1999), New Zealand brill (Diggles et al. 2000), carp, and trout, with mechanisms involving adhesion, biofilm formation, and toxin secretion (Roux and Blokesch 2018; Letchumanan, Chan, and Lee 2014). However, marbled flounder may be more susceptible due to slower epithelial repair and immune response under environmental stress (Shi et al. 2023).
Environmental factors such as water temperature, salinity, and organic load significantly affect V. splendidus pathogenicity. Infections are often associated with temperatures above 15 °C (Thomson et al. 2005; Austin and Austin 2016), and salinity (2–8%) and dissolved organic carbon enhance biofilm and virulence expression (Eiler, Johansson, and Bertilsson 2006; Oyanedel et al. 2020). High-density culture, poor water quality, and skin damage from handling or parasitism increase infection risk (Spanggaard et al. 2000; Kokou et al. 2020; Sobhana et al. 2009). Rapid bacterial growth observed in this study (peaking within 10–12 h) suggests that early intervention post-injury is critical for disease control.
Antibiotic sensitivity tests showed that quinolones (enrofloxacin, ciprofloxacin) and amides (florfenicol) were effective against all isolates, while resistance to erythromycin and amoxicillin was widespread. Doxycycline showed good activity, whereas sensitivity to ceftazidime and sulfadiazine-trimethoprim varied. These findings highlight the need for treatment based on specific susceptibility profiles (Zanetti et al. 2001). Monitoring resistance patterns and determining MIC values in future studies will help optimize therapeutic regimens.
Given concerns over antibiotic resistance, alternative strategies such as probiotics and improved husbandry should be integrated into disease management (Hai 2015; Chauhan and Singh 2019). However, this study has limitations: it captures only a snapshot in time and may not reflect long-term pathogen dynamics or geographic variation (Ke Huei et al. 2017) Liang et al., 2022; Lion and Metz 2018). Therefore, multi-year studies across broader environments are necessary to generalize these findings.
5. Conclusions
In summary, Vibrio splendidus infection causes skin ulcers and varying degrees of lesions in the kidneys, intestines, gills, and liver. However, the severity of the disease under laboratory conditions was significantly lower than that in natural artificial breeding environments. Moreover, in the natural environment, the probability of disease in P. yokohamae is significantly lower than that in cultured environments. The disease may be triggered by V. splendidus infection and exacerbated by factors such as overcrowding, handling, environmental fluctuations, biofouling, and predator interactions, all of which collectively induce stress in fish.